Epidemiology, disease burden and costs of Duchenne muscular dystrophy in Germany: an observational, retrospective health claims data analysis
- PMID: 40804403
- PMCID: PMC12351880
- DOI: 10.1186/s13023-025-03906-x
Epidemiology, disease burden and costs of Duchenne muscular dystrophy in Germany: an observational, retrospective health claims data analysis
Abstract
Background: Duchenne muscular dystrophy (DMD) is a rare genetic disorder that primarily affects males. Beginning in childhood, patients experience ambulatory loss, heart failure and need ventilation. Disease management has improved, however, DMD remains debilitating, and has no cure. The rarity of the disease makes research difficult, and German prevalence data are lacking. Cost and resource utilization estimations are based on small sample sizes or self-reported data, limiting generalizability and adds the potential for recall bias. With a retrospective study on a healthcare claims database, we adapted algorithms to identify DMD patients and categorized them by disease stages 1-4 (early ambulatory, late ambulatory, early non-ambulatory, late non-ambulatory) with increasing disease progression. We analyzed annual prevalence, burden of disease, healthcare resource utilization and direct medical care costs, by time under observation (patient year).
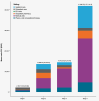
Results: From 2016 to 2021, we identified 134 patients for which we could determine a disease stage and determined an extrapolated prevalence of DMD in Germany between 14.85 (95%CI 12.17, 17.95) and 18.91 (95%CI 15.70, 22.61) per 100,000 males under 40 years of age. Most patients we identified met DMD stage 4 group criteria (47.01%), followed by stage 3 (37.31%), stage 2 (33.58%) and only 4.48% in stage 1. The average age increased with progressing disease, from 4.27 years in stage 1, to 11.43, 18.86 and 23.21 in stage groups 2, 3 and 4, respectively. In the stage 2 group, diagnosis codes reflecting mobility support and orthopedic surgical interventions (15.56% of the group) were documented. In the stage 3 group, decubitus prevention was documented, increasing to around half of patients in the stage 4 group. Total direct mean healthcare costs per patient year increased substantially with disease severity group, from €2,180.73 (SD 16,258.90) in stage 1; €13,599.83 (SD 33,756.07) in stage 2; €14,472.08 (SD 27,245.78) in stage 3 and finally €41,888.70 (SD 117,718.13) in stage 4. Especially in stage groups 3 and 4, medical aids accounted for about half of total costs.
Conclusions: We present an algorithm on which further research can be based, and provide a current picture of epidemiology, burden of disease and healthcare utilization and direct costs of DMD in Germany.
Keywords: Algorithm; Costs; Disease stages; Duchenne muscular dystrophy; Epidemiology; Germany; Healthcare resource utilization; Prevalence; Real-world data.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: JD, TG, and CM are employees of WIG2 GmbH. JKö, LK, CS, IH and AK are employed by Pfizer Pharma GmbH. JKi has received grants for consultancy and educational activities from Italfarmaco, Pfizer, Roche, Santhera, and Sarepta. AP reports financial compensation for training activities and research grants from Novartis and Biogen. The authors report no other conflicts of interest in this work. Ethical approval and consent to participate: No primary patient data was collected for the conduct of this research. According to the 10th chapter of book V of social code in Germany (SGB V, Sozialgesetzbuch), anonymized healthcare claims data can be used for research purposes, therefore ethics approval of this secondary data were not required. Only WIG2 employees were granted access to the data for the research, and only aggregated results were generated and presented. Consent for publication: This work has not been published previously, and it is not under consideration for publication elsewhere. If accepted, it will not be published elsewhere in the same form in English or in any other language including electronically, without the written consent of the copyright holder. The publication and all data, visualizations and content have been approved for publication by all authors.
Figures



Similar articles
-
The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review.Orphanet J Rare Dis. 2017 Apr 26;12(1):79. doi: 10.1186/s13023-017-0631-3. Orphanet J Rare Dis. 2017. PMID: 28446219 Free PMC article.
-
Antioxidants to prevent respiratory decline in people with Duchenne muscular dystrophy and progressive respiratory decline.Cochrane Database Syst Rev. 2021 Dec 1;12(12):CD013720. doi: 10.1002/14651858.CD013720.pub3. Cochrane Database Syst Rev. 2021. PMID: 34850383 Free PMC article.
-
Antioxidants to prevent respiratory decline in people with Duchenne muscular dystrophy and progressive respiratory decline.Cochrane Database Syst Rev. 2021 Nov 8;11(11):CD013720. doi: 10.1002/14651858.CD013720.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2021 Dec 1;12:CD013720. doi: 10.1002/14651858.CD013720.pub3. PMID: 34748221 Free PMC article. Updated.
-
[Administrative healthcare data to identify and describe patients with rare diseases: the case of Duchenne muscular dystrophy].Recenti Prog Med. 2025 May;116(5):310-321. doi: 10.1701/4495.44951. Recenti Prog Med. 2025. PMID: 40376903 Italian.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
References
-
- Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251–67. 10.1016/S1474-4422(18)30024-3. - PMC - PubMed
-
- Rubin M. Duchenne-Muskeldystrophie und Becker-Muskeldystrophie: Ausgabe für medizinische Fachkreise. 2022. https://www.msdmanuals.com/de-de/profi/p%C3%A4diatrie/angeborene-muskelk.... Accessed 01/2022.
-
- Orphanet: Das Portal für seltene Krankheiten und Orphan Drugs. Muskeldystrophie Typ Duchenne. https://www.orpha.net/de/disease/detail/98896#menu. Accessed November 2024.
-
- Brabec P, Vondrácek P, Klimes D, Baumeister S, Lochmüller H, Pavlík T, Gregor J. Characterization of the DMD/BMD patient population in Czech Republic and Slovakia using an innovative registry approach. Neuromuscul Disord. 2009;19:250–4. 10.1016/j.nmd.2009.01.005. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

