Development of an innovative duplex digital PCR assay for circulating MiRNA ratio quantification in metastatic melanoma
- PMID: 40804405
- PMCID: PMC12351995
- DOI: 10.1186/s12967-025-06941-1
Development of an innovative duplex digital PCR assay for circulating MiRNA ratio quantification in metastatic melanoma
Abstract
Background: Circulating miRNAs (cmiRNAs) are emerging as valuable non-invasive biomarkers for monitoring disease progression and therapeutic response in cancer. Their stability in biological fluids, tissue-specific expression, and functional roles in tumor biology make them particularly suitable for liquid biopsy approaches. However, challenges related to quantification accuracy and assay standardization have limited their clinical translation. Digital PCR (dPCR) offers a highly sensitive and reproducible solution for absolute quantification of low-abundance transcripts, addressing many of these limitations.
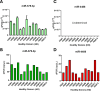
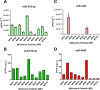
Methods: We developed and analytically validated the first duplex dPCR assay for the simultaneous detection of miR-4488 and miR-579-3p in serum samples from patients with BRAF-mutant metastatic melanoma receiving MAPK inhibitor therapy. These two cmiRNAs were previously identified by our group as biomarkers predictive of treatment response. Using fluorescently labelled probes, both targets were co-amplified in a single reaction. The assay was tested for analytical performance, including comparison with singleplex formats and quantitative Real-Time PCR (qRT-PCR). We then applied the duplex assay to assess the prognostic potential of the expression ratio between the two miRNAs, termed miRatio, at baseline and over treatment timepoints.
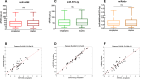
Results: The duplex assay maintained analytical performance comparable to singleplex reactions while reducing sample and reagent use. Compared to qRT-PCR, dPCR showed superior sensitivity, particularly for detecting low-abundance miRNAs like miR-4488. miRatio effectively predicts disease outcome when measured at baseline prior to MAPKi therapy and exhibits dynamic changes during treatment, supporting its potential as a longitudinal biomarker. ROC analysis demonstrated strong prognostic value, with improved accuracy over previous qRT-PCR-based evaluations.
Conclusions: This study highlights duplex dPCR as a robust, sensitive, and scalable technology for circulating miRNA quantification in liquid biopsy applications. By enabling absolute and simultaneous detection of miR-4488 and miR-579-3p, the assay provides a technically advanced platform for real-time monitoring in metastatic melanoma. While miRatio remains a promising biomarker, the key innovation of this work is the development of a duplex assay suitable for clinical implementation in precision oncology.
Keywords: Circulating MiRNAs; Digital PCR; Duplex assay; Liquid biopsy; Metastatic melanoma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committees of both participating institutions: the IRCCS “G. Pascale Foundation” (Approval Numbers DSC/1504, June 11, 2014 and DSC/2893, April 11, 2015) and the IRCCS Regina Elena National Cancer Institute-IFO (Approval Number 8393, July 23, 2017), in accordance with the Declaration of Helsinki. Inclusion criteria specified patients aged ≥ 18 years with histologically confirmed, locally advanced or metastatic melanoma (Stage IIIB-IIIC or IV according to the American Joint Committee on Cancer, 7th Edition), eligible for MAPK-targeted therapy, and capable of providing informed consent. Samples from healthy donors were collected with the approval of the IRCCS Regina Elena National Cancer Institute (Rome, Italy) institutional ethics committee (Approval Number 7182, May 24, 2019). Written informed consent was obtained from all participants before inclusion in the study. Consent for publication: All the authors agreed for publication of this research. Competing interests: All the authors declare no conflict of interest with the exceptions of P.A.A. P.A.A. has/had a consultant/advisory role for Bristol MyersSquibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-Al Health, ValoTX, Replimmune. He also received research funding from Bristol Myers Squibb, Roche-Genentech, Pfizer, Sanofi. Travel support by Pfizer. The funders of this study had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Challenges in circulating miRNA analysis in adrenocortical tumors.Endocr Relat Cancer. 2025 Jun 17;32(6):e250045. doi: 10.1530/ERC-25-0045. Print 2025 Jun 1. Endocr Relat Cancer. 2025. PMID: 40472370
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Serum extracellular vesicle microRNAs as potential biomarkers to predict pembrolizumab response and prognosis in metastatic non-small cell lung cancer patients.Front Immunol. 2025 Jun 4;16:1540906. doi: 10.3389/fimmu.2025.1540906. eCollection 2025. Front Immunol. 2025. PMID: 40534853 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Alix-Panabières C, Pantel K. Advances in liquid biopsy: from exploration to practical application. Cancer Cell. 2025;43(2):161–5. - PubMed
-
- Monette A, Aguilar-Mahecha A, Altinmakas E, Angelos MG, Assad N, Batist G, et al. The society for immunotherapy of cancer perspective on liquid biopsy and radiomics based technologies for immuno-oncology biomarker discovery and application. Clin Cancer Res. 2025. 10.1158/1078-0432.CCR-24-3791. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

