Study protocol testing feasibility of the Comfort Measures Only Time out (CMOT) to reduce distress during palliative withdrawal of mechanical ventilation
- PMID: 40804432
- PMCID: PMC12345127
- DOI: 10.1186/s40814-025-01688-4
Study protocol testing feasibility of the Comfort Measures Only Time out (CMOT) to reduce distress during palliative withdrawal of mechanical ventilation
Abstract
Introduction: Distress is experienced by more than 30% of patients during palliative withdrawal of mechanical ventilation at the end of life in the intensive care unit. There is a lack of high-quality evidence for specific approaches to risk factor identification and management of distress during this process. Structured "time-outs" and checklist interventions improve surgical outcomes and have been widely adopted in procedural care, but they have not been tested for use at end-of-life in intensive care unit settings.
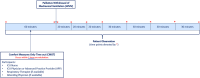
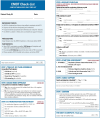
Methods: We describe the development and planned testing of a novel time-out checklist intervention, the Comfort Measures Only Time Out (CMOT) in a non-randomized single arm pilot study. Intervention development was guided by published literature and a structured inter-professional advisory panel. The intervention will be tested by clinical teams caring for 46 patients undergoing palliative withdrawal of mechanical ventilation. Nurses, physicians, advanced practice providers, and respiratory therapists will convene within an hour before withdrawal of mechanical ventilation to complete the checklist. Implementation outcomes, including feasibility, will be measured by a 12-question survey and by clinician protocol adherence. Effect size calculations will determine power for future randomized controlled trials testing efficacy of the CMOT in reducing patient distress.
Discussion: This protocol will pilot test the feasibility of the CMOT, a structured time-out and checklist intervention, for WMV in the ICU. The study will inform potential changes to the protocol and intervention for a future randomized control trial. The CMOT is grounded in a quality and safety framework already adopted in procedural and critical care settings. Given high rates of distress, the CMOT will fill an identified gap in evidence surrounding the process of WMV.
Trial registration: Clinical trials.gov ( NCT05861323 ); 16 May 2023.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Beth Israel Deaconess Medical Center Committee on Clinical Investigations (CCI/IRB) approved this study (#2023P000160) and will review any protocol changes. The trial is registered on ClinicalTrials.gov (NCT05861323) and with the National Institute on Aging CROMS database (DGCG-10874). Participants will be assigned a unique identifier to protect confidentiality. All study databases will be de-identified and archived at the end of the study. All data are encrypted and stored in secure, password protected research virtual environment accessible only to approved study staff. Consent for publication: All authors read and approved the final manuscript. Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Extracorporeal carbon dioxide removal for the treatment of acute hypoxaemic respiratory failure: the REST RCT.Health Technol Assess. 2025 Jul;29(33):1-16. doi: 10.3310/GJDM0320. Health Technol Assess. 2025 Jul;29(33):1-16. doi: 10.3310/GJDM0320. PMID: 40758387 Clinical Trial.
-
Interventions for interpersonal communication about end of life care between health practitioners and affected people.Cochrane Database Syst Rev. 2022 Jul 8;7(7):CD013116. doi: 10.1002/14651858.CD013116.pub2. Cochrane Database Syst Rev. 2022. PMID: 35802350 Free PMC article.
-
Exercise rehabilitation following intensive care unit discharge for recovery from critical illness.Cochrane Database Syst Rev. 2015 Jun 22;2015(6):CD008632. doi: 10.1002/14651858.CD008632.pub2. Cochrane Database Syst Rev. 2015. PMID: 26098746 Free PMC article.
-
Palliative care interventions in advanced dementia.Cochrane Database Syst Rev. 2016 Dec 2;12(12):CD011513. doi: 10.1002/14651858.CD011513.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Sep 28;9:CD011513. doi: 10.1002/14651858.CD011513.pub3. PMID: 27911489 Free PMC article. Updated.
References
-
- Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–43. - PubMed
-
- Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506–14. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

