Effectiveness of remdesivir for hospitalized COVID-19 patients depending on the severity of respiratory status: a multicenter retrospective study in Japan
- PMID: 40804665
- PMCID: PMC12345095
- DOI: 10.1186/s12879-025-11345-z
Effectiveness of remdesivir for hospitalized COVID-19 patients depending on the severity of respiratory status: a multicenter retrospective study in Japan
Abstract
Background: Remdesivir, an antiviral nucleotide analog prodrug, is approved for clinical use against COVID-19 worldwide. However, its effectiveness varies depending on the respiratory failure status of patients. This study aimed to evaluate the effectiveness of remdesivir treatment based on the severity of respiratory failure, as indicated by the oxygen demand upon hospital admission. Subgroups analyses were conducted to identify patient groups that might benefit from remdesivir treatment.
Methods: This retrospective observational study (the J-RECOVER) enrolled patients with COVID-19 from 64 institutions in Japan between January 1 and September 30, 2020. Patients aged ≥ 18 years who were administered remdesivir within 3 days of hospital admission were included. A total of 3,591 patients were included, and propensity score overlap weighting analysis was used to compare in-hospital mortality based on respiratory failure status at admission between remdesivir and control groups. Subgroup analyses identified specific patient populations that may benefit most from remdesivir treatment, considering factors such as respiratory status and renal function.
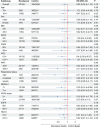
Results: The overlap weighting (OW)-adjusted odds ratio (OR) for mortality in overall cohort, mild cases without supplemental oxygen, moderate cases requiring supplemental oxygen, and severe cases requiring ventilation was (OR, 0.65 (95% confidence interval (CI), 0.36-1.19; P = 0.16), 0.11 (95% CI, 0.01-1.03; P = 0.05). 0.82 (95% CI, 0.31-2.16; P = 0.69), and 0.78 (95% CI, 0.28-2.17; P = 0.63), respectively. A trend toward improvement in mortality was observed in respiratory indicators, such as SpO2 ≥ 94% (OR, 0.43; 95% CI, 0.19-0.99; P = 0.04), oxygen support with FiO2 < 0.5 (OR, 0.40; 95% CI, 0.16-0.97; P = 0.04), and PFR ≥ 300 (OR, 0.17; 95% CI, 0.03-0.94; P = 0.04). Subgroup analyses indicated improved mortality in patients with an estimated glomerular filtration rate (eGFR) of > 60 mL/min per 1.73 m2 (OR, 0.29; 95% CI, 0.09-0.94; P = 0.03), with a p-value for interaction of P = 0.18.
Conclusion: Remdesivir treatment may reduce the risk of in-hospital mortality in patients with mild respiratory distress. Subgroup analysis suggested that remdesivir treatment may improve mortality in patients with eGFR ≥ 60 mL/min per 1.73 m2.
Keywords: COVID-19; Japan; Overlap weighting; Propensity score; Remdesivir; Respiratory status.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (registration no. UMIN000047056). The registration date for this study was March 2, 2022. This study was approved by the Institutional Review Board of Nippon Medical School Musashikosugi Hospital (approval no. 561-2-26), which waived the requirement for informed consent because of the anonymous nature of retrospective data. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- World Health Organization. COVID-19 weekly epidemiological update Edition 98. 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

