6-year treatment follow-up with an extended-release alkaline formulation (Sibnayal®) in primary distal renal tubular acidosis
- PMID: 40804680
- PMCID: PMC12351860
- DOI: 10.1186/s13023-025-03953-4
6-year treatment follow-up with an extended-release alkaline formulation (Sibnayal®) in primary distal renal tubular acidosis
Abstract
Background: Distal renal tubular acidosis (dRTA) is a rare disease characterized by hyperchloremic metabolic acidosis affecting growth, bone and kidney health.
Methods: The aim of B22CS study was to evaluate long-term safety and efficacy (anthropometric/pubertal, tubular damages/kidney function, bone biomarkers, compliance assessments) of Sibnayal®, a prolonged-release alkalinizing formulation with twice daily dosing, in children and adults with dRTA. All patients were previously included in the pivotal B21CS study, so were already receiving Sibnayal® when included in B22CS open-label follow-up study.
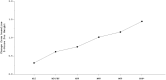
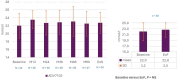
Results: A total of 30 patients with primary dRTA (mean age:10.6 ± 6.0 years) entered this long-term study (average of 6 years). At inclusion, most patients had adequate metabolic control, normal kidney function and height. Sibnayal® was well tolerated over the study duration.The most frequent adverse event was hypovitaminosis D (13 patients). Causality to treatment was reported for only 4% of all TEAEs (6 patients) and were mostly gastrointestinal. All adverse events resolved without treatment discontinuation. Sibnayal® allowed a sustained control of metabolic acidosis as plasma bicarbonate level was 22.0 ± 3.2 mmol/L at baseline versus 22.6 ± 2.5 mmol/L at the End of Follow-up (EoF), p = NS. From baseline to EoF, mean Z-score height significantly increased (-0.6 ± 1.0 to -0.3 ± 1.0, p = 0.03), without significant change in weight and body mass index. Kidney function remained stable from baseline to EoF: estimated glomerular filtration rate = 105 ± 17 and 104 ± 20 mL/min/1.73m2, respectively, p = NS. Urinary ratios: Calcium/Creatinine (UCa/UCr), Citrate/Creatinine (UCi/UCr), Calcium/Citrate (UCa/UCi) were not significantly different between baseline and EoF (p = NS). Mean lumbar bone mineral density Z-score significantly increased from baseline (-1.1 ± 1.0) to EoF (-0.8 ± 1.0), p = 0.005, with significant improvement between baseline and EoF in pre- and post-pubertal patients (p = 0.035 and p < 0.001, respectively), whilst it was maintained in pubertal patients (p = NS).
Conclusion: Long-term data support the good safety and efficacy profile of Sibnayal® in the treatment of dRTA with adequate control of metabolic acidosis, stable kidney function and significant positive long-term clinical outcomes.
Key learning points: WHAT WAS KNOWN: The medical management of dRTA consists in the correction of metabolic acidosis to prevent long-term complications on growth, bone metabolism and kidney function. Growth and kidney function were shown to be correlated to metabolic control. Only half of patients had adequate metabolic control. More than 80% of adult patients had CKD KDIGO ≥ 2.
This study adds: For the first time, a long-term follow-up study provides safety and efficacy data of Sibnayal®, an alkali medication with long-lasting release allowing 2 intakes per day. This study supports a long-term adequate control of metabolic acidosis with Sibnayal®, along with significant clinical outcomes on growth, kidney function and bone metabolism.
Potential impact: The main challenge for physicians is to prevent long-term complications of dRTA by better controlling metabolic acidosis. Sibnayal® as an innovative prolonged-release alkalinizing formulation with twice daily dosing, adapted for children, contributes to improving current medical management, clinical outcomes and quality of life of dRTA patients.
Trial registration number: Registered as EudraCT 2013-003828-36 on September 03, 2013.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study was approved by regional independent ethics committees (CPP sud-est II; clinical center of niš, ethics committee, Niš; Etická komisia Detská fakultná nemocnica s poliklinikou Bratislava, Bratislava) and national regulatory health authorities and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. Consent to participate: Written informed consent was obtained from all adult patients, and from the parents or legal guardians of all children. When possible, the assent from pediatric patients themselves was also obtained. Consent for publication: All authors consent for publication. Competing interests: A. Bertholet-Thomas received travel and speaker fees from Advicenne. J. Bacchetta received speaker fees from Advicenne. L. Chidler and V. Leblanc are Advicenne’s employees.
Figures





References
-
- Trepiccione F, Walsh SB, Ariceta G, et al. Distal renal tubular acidosis: erknet/espn clinical practice points. Nephrol Dial Transpl. 2021;36(9):1585–96. 10.1093/ndt/gfab171. - PubMed
-
- Vallés PG, Batlle D. Hypokalemic distal renal tubular acidosis. Adv Chronic Kidney Dis. 2018;25(4):303–20. 10.1053/j.ackd.2018.05.003. - PubMed
-
- Gómez-Conde S, García-Castaño A, Aguirre M, et al. Molecular aspects and long-term outcome of patients with primary distal renal tubular acidosis. Pediatr Nephrol. 2021;36(10):3133–42. 10.1007/s00467-021-05066-z. - PubMed
-
- Forero-Delgadillo JM, Gil-Peña H, Alonso-Varela M, Santos F, RenalTube G. Kidney function in patients with primary distal renal tubular acidosis. Pediatr Nephrol. 2021;36(7):1931–5. 10.1007/s00467-021-05068-x. - PubMed
-
- Lopez-Garcia SC, Emma F, Walsh SB, et al. Treatment and long-term outcome in primary distal renal tubular acidosis. Nephrol Dial Transpl. 2019;34(6):981–91. 10.1093/ndt/gfy409. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

