Melatonin Synergises the Chemotherapeutic Effect of Temozolomide in Glioblastoma by Suppressing NF-κB/COX-2 Signalling Pathways
- PMID: 40804775
- PMCID: PMC12350191
- DOI: 10.1111/jcmm.70778
Melatonin Synergises the Chemotherapeutic Effect of Temozolomide in Glioblastoma by Suppressing NF-κB/COX-2 Signalling Pathways
Abstract
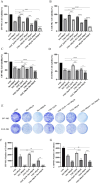
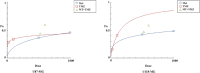
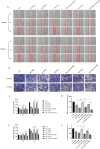
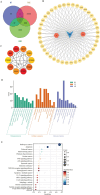
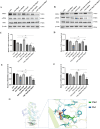
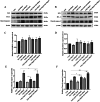
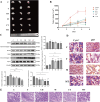
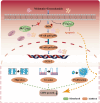
Glioblastoma (GBM) is an aggressive and highly malignant primary brain tumour, accounting for a significant proportion of adult brain tumours. It is associated with a poor prognosis and high recurrence rates. Although temozolomide (TMZ) remains the standard first-line chemotherapy for GBM, its clinical efficacy is often limited by the development of drug resistance and toxic effects on normal tissues. Melatonin (Mel), a natural indoleamine synthesised by the pineal gland, has demonstrated synergistic anti-tumour effects when combined with various chemotherapy agents in multiple studies. This study investigates the synergistic potential of Mel to enhance TMZ's therapeutic efficacy against GBM. The results demonstrate that the combination of Mel and TMZ significantly inhibits glioblastoma cell proliferation, migration, and invasion. Mechanistically, this synergistic effect is mediated through the NF-κB/COX-2 signalling pathway. Mel enhances TMZ's anti-tumour activity by inhibiting IκBα phosphorylation, suppressing NF-κB activation, and downregulating COX-2 expression. Additionally, the combination treatment induced apoptosis via activation of the Caspase-3 pathway. These results suggest that Mel can potentiate the therapeutic efficacy of TMZ in glioblastoma treatment, offering a promising strategy to overcome TMZ resistance while reducing its associated toxicity.
Keywords: NF‐κB/COX‐2; glioblastoma; melatonin; temozolomide.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The HBP Pathway Inhibitor FR054 Enhances Temozolomide Sensitivity in Glioblastoma Cells by Promoting Ferroptosis and Inhibiting O-GlcNAcylation.CNS Neurosci Ther. 2025 Aug;31(8):e70546. doi: 10.1111/cns.70546. CNS Neurosci Ther. 2025. PMID: 40772310 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Intranasal delivery of temozolomide and desloratadine for brain tumour therapy: A cellular study on nasal epithelial toxicity, transport, and permeability.J Pharm Sci. 2025 Jul;114(7):103795. doi: 10.1016/j.xphs.2025.103795. Epub 2025 Apr 14. J Pharm Sci. 2025. PMID: 40239838
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
References
-
- Tan A. C., Ashley D. M., López G. Y., Malinzak M., Friedman H. S., and Khasraw M., “Management of Glioblastoma: State of the Art and Future Directions,” CA: A Cancer Journal for Clinicians 70, no. 4 (2020): 299–312. - PubMed
-
- Stepanovic A. and Nikitovic M., “Severe Hematologic Temozolomide‐Related Toxicity and Lifethreatening Infections,” Journal of B.U.ON.: Official Journal of the Balkan Union of Oncology 23, no. 1 (2018): 7–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

