Fast Evolving Glioblastoma in a Pregnant Woman: Diagnostic and Therapeutic Challenges
- PMID: 40804801
- PMCID: PMC12345949
- DOI: 10.3390/diagnostics15151836
Fast Evolving Glioblastoma in a Pregnant Woman: Diagnostic and Therapeutic Challenges
Abstract
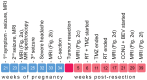
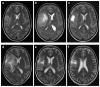
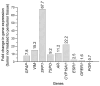
Background and Clinical Significance: Gliomas diagnosed during pregnancy are rare, and there are no established guidelines for their management. Effective treatment requires a multidisciplinary approach to balance maternal health and pregnancy preservation. Case Presentation: We here present a case of rapidly progressing glioma in a 33-year-old pregnant woman. The patient initially presented with a generalized tonic-clonic seizure at 21 weeks' gestation. Imaging revealed a tumor in the right cerebral lobe, involving both cortical and subcortical structures, while magnetic resonance spectroscopy suggested a low-grade glioma. The patient remained clinically stable for two months but then developed severe headaches; MRI showed a worsening mass effect. At 34 weeks' gestation, an emergency and premature caesarean section was performed under general anesthesia. The patient then underwent a craniotomy for maximal tumor resection, which was histologically and molecularly diagnosed as IDH wild-type glioblastoma (GB). Using qPCR, we found that the GB tissue showed upregulated expression of genes involved in cell structure (GFAP, VIM) and immune response (SSP1, TSPO), as well as increased expression of genes related to potential hormone response (AR, CYP19A1, ESR1, GPER1). After surgery, the patient showed resistance to Stupp protocol therapy, which was substituted with lomustine and bevacizumab combination therapy. Conclusions: This case illustrates that glioma may progress rapidly during pregnancy, but a favorable obstetric outcome is achievable. Management of similar cases should respect both the need for timely treatment and the patient's informed decision.
Keywords: brain cancer; glioblastoma therapy; magnetic resonance spectroscopy; maternal–fetal outcome; pregnancy.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants.Cochrane Database Syst Rev. 2018 Jan 5;1(1):CD012910. doi: 10.1002/14651858.CD012910. Cochrane Database Syst Rev. 2018. PMID: 29303230 Free PMC article.
-
Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome.Cochrane Database Syst Rev. 2017 Mar 3;3(3):CD004735. doi: 10.1002/14651858.CD004735.pub4. Cochrane Database Syst Rev. 2017. PMID: 28257562 Free PMC article.
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
References
-
- Norris J.N., Waack A.L., Becker K.N., Keener M., Hoyt A., Reinard K. Glioblastoma in pregnant patient with pathologic and exogenous sex hormone exposure and family history of high-grade glioma: A case report and review of the literature. Surg. Neurol. Int. 2023;14:169. doi: 10.25259/SNI_58_2023. - DOI - PMC - PubMed
-
- Peeters S., Pagès M., Gauchotte G., Miquel C., Cartalat-Carel S., Guillamo J.S., Capelle L., Delattre J.Y., Beauchesne P., Debouverie M., et al. Interactions between glioma and pregnancy: Insight from a 52-case multicenter series. J. Neurosurg. 2018;128:3–13. doi: 10.3171/2016.10.JNS16710. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

