Saliva Has High Sensitivity and Specificity for Detecting SARS-CoV-2 Compared to Nasal Swabs but Exhibits Different Viral Dynamics from Days of Symptom Onset
- PMID: 40804882
- PMCID: PMC12346843
- DOI: 10.3390/diagnostics15151918
Saliva Has High Sensitivity and Specificity for Detecting SARS-CoV-2 Compared to Nasal Swabs but Exhibits Different Viral Dynamics from Days of Symptom Onset
Abstract
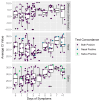
Background/Objectives: Saliva as a diagnostic medium for COVID-19 requires fewer resources to collect and is more readily adopted across a range of testers. Our study compared an Emergency Use Authorized direct saliva-to-RT-qPCR test against an FDA-authorized nasal swab RT-qPCR assay for participants who reported symptoms of respiratory infection. Methods: We analyzed 737 symptomatic participants who self-selected to test at either a community testing facility or a walk-in clinic due to respiratory symptoms and provided matched saliva and nasal swab samples. Samples were collected between March and September of 2023, both before and after the declared end of the public health emergency. Results: A total of 120 participants tested positive in at least one of the tests. For participants testing in the first 5 days of reported symptoms, the saliva test had a 94.0 positive percent agreement (PPA; 95% C.I. 88.9-99.1%) with the nasal test and a 99.0 negative percent agreement (NPA; 95% C.I. 98.1-99.9%). The viral load decreased beyond day 1 of reported symptoms for saliva testing. Viral load increased up to day 4 for nasal swabs and then decreased. The same number of discordant positive samples (five each) occurred for both tests within 5 days of symptoms onset. Conclusions: In the endemic phase of COVID-19 and for development of new tests, testing methods that are less invasive are more likely to be adopted. The results of saliva-based versus nasal swab PCR measurements relative to days of symptom onset are needed to optimize future testing strategies.
Keywords: COVID-19; SARS-CoV-2; diagnostic; qPCR; saliva.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Kandel C.E., Young M., Serbanescu M.A., Powis J.E., Bulir D., Callahan J., Katz K., McCready J., Racher H., Sheldrake E., et al. Detection of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) in outpatients: A multicenter comparison of self-collected saline gargle, oral swab, and combined oral-anterior nasal swab to a provider collected nasopharyngeal swab. Infect. Control. Hosp. Epidemiol. 2021;42:1340–1344. doi: 10.1017/ice.2021.2. - DOI - PMC - PubMed
-
- Kim Y.G., Yun S.G., Kim M.Y., Park K., Cho C.H., Yoon S.Y., Nam M.H., Lee C.K., Cho Y.-J., Lim C.S. Comparison between Saliva and Nasopharyngeal Swab Specimens for Detection of Respiratory Viruses by Multiplex Reverse Transcription-PCR. J. Clin. Microbiol. 2016;55:226–233. doi: 10.1128/JCM.01704-16. - DOI - PMC - PubMed
-
- To K.K.W., Chan K.H., Ho J., Pang P.K.P., Ho D.T.Y., Chang A.C.H., Seng C.W., Yip C.C.Y., Cheng V.C.C., Hung I.F.N., et al. Respiratory virus infection among hospitalized adult patients with or without clinically apparent respiratory infection: A prospective cohort study. Clin. Microbiol. Infect. 2019;25:1539–1545. doi: 10.1016/j.cmi.2019.04.012. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous