Molar Pregnancy: Early Diagnosis, Clinical Management, and the Role of Referral Centers
- PMID: 40804917
- PMCID: PMC12346673
- DOI: 10.3390/diagnostics15151953
Molar Pregnancy: Early Diagnosis, Clinical Management, and the Role of Referral Centers
Abstract
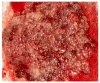
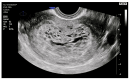
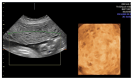
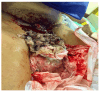
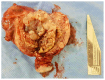
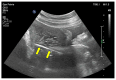
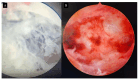
Molar pregnancy (MP) is a gestational disorder resulting from abnormal fertilization, leading to atypical trophoblastic proliferation and the formation of a complete or partial hydatidiform mole. This condition represents the most common form of gestational trophoblastic disease (GTD) and carries a significant risk of progression to gestational trophoblastic neoplasia (GTN). Although rare in high-income countries, MP remains up to ten times more prevalent in low-income and developing countries, contributing to preventable maternal morbidity and mortality. This narrative review provides an updated, practical overview of the clinical presentation, diagnosis, treatment, and follow-up of MP. A key focus is the challenge of early diagnosis, particularly given the increasing frequency of first-trimester detection, where classical histopathological criteria may be subtle, leading to diagnostic errors. The review innovates by integrating advanced diagnostic methods-combining histopathology, immunohistochemistry using p57Kip2, Ki-67, and p53 markers, along with cytogenetic analysis-to improve diagnostic accuracy in early gestation. The central role of referral centers is also emphasized, not only in facilitating timely treatment and access to chemotherapy, but also in implementing standardized post-molar follow-up protocols that reduce progression to GTN and maternal mortality. By focusing on both advanced diagnostic strategies and the organization of care through referral centers, this review offers a comprehensive, practice-oriented perspective to optimize patient outcomes in GTD and address persistent care gaps in high-burden regions.
Keywords: clinical presentation; diagnosis; follow-up; molar pregnancy; treatment.
Conflict of interest statement
The authors report no conflict of interest.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia.Cochrane Database Syst Rev. 2017 Sep 11;9(9):CD007289. doi: 10.1002/14651858.CD007289.pub3. Cochrane Database Syst Rev. 2017. PMID: 28892119 Free PMC article.
-
Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia.Cochrane Database Syst Rev. 2012 Oct 17;10(10):CD007289. doi: 10.1002/14651858.CD007289.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Sep 11;9:CD007289. doi: 10.1002/14651858.CD007289.pub3. PMID: 23076934 Free PMC article. Updated.
-
Immunohistochemical Staining: Prognostic Marker of Malignant Transformation of Hydatidiform Mole (HM).J Obstet Gynaecol India. 2025 Apr;75(Suppl 1):365-370. doi: 10.1007/s13224-024-02002-7. Epub 2024 Jul 23. J Obstet Gynaecol India. 2025. PMID: 40390880
-
Ophthalmia Neonatorum.2025 Jul 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31855399 Free Books & Documents.
References
-
- Lok C., van Trommel N., Braicu E.I., Planchamp F., Berkowitz R., Seckl M., EOTTD-ESGO-GCIG-ISSTD Guideline Committee Practical Guidelines for the Treatment of Gestational Trophoblastic Disease: Collaboration of the European Organisation for the Treatment of Trophoblastic Disease (EOTTD)-European Society of Gynaecologic Oncology (ESGO)-Gynecologic Cancer InterGroup (GCIG)-International Society for the Study of Trophoblastic Diseases (ISSTD) J. Clin. Oncol. 2025;43:2119–2128. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

