Multidisciplinary, Clinical Assessment of Accelerated Deep-Learning MRI Protocols at 1.5 T and 3 T After Intracranial Tumor Surgery and Their Influence on Residual Tumor Perception
- PMID: 40804946
- PMCID: PMC12346804
- DOI: 10.3390/diagnostics15151982
Multidisciplinary, Clinical Assessment of Accelerated Deep-Learning MRI Protocols at 1.5 T and 3 T After Intracranial Tumor Surgery and Their Influence on Residual Tumor Perception
Abstract
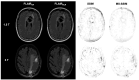
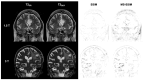
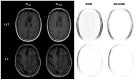
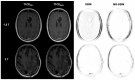
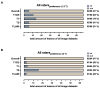
Background/Objectives: Postoperative MRI is crucial for detecting residual tumor, identifying complications, and planning subsequent therapy. This study evaluates accelerated deep learning reconstruction (DLR) versus standard clinical protocols for early postoperative MRI following tumor resection. Methods: This study uses a multidisciplinary approach involving a neuroradiologist, neurosurgeon, neuro-oncologist, and radiotherapist to evaluate qualitative aspects using a 5-point Likert scale, the preferred reconstruction variant and potential residual tumor of DLR and conventional reconstruction (CR) of FLAIR, T1-weighted non-contrast and contrast-enhanced (T1), and coronal T2-weighted (T2) sequences for 1.5 and 3 T MRI. Quantitative analysis included the image quality metrics Structural Similarity Index (SSIM), Multi-Scale SSIM (MS-SSIM), Feature Similarity Index (FSIM), Noise Quality Metric (NQM), signal-to-noise ratio (SNR), and Peak SNR (PSNR) with CR as a reference. Results: All raters strongly preferred DLR over CR. This was most pronounced for FLAIR images at 1.5 and 3 T (91% at 1.5 T and 97% at 3 T) and least pronounced for T1 at 1.5 T (79% for non-contrast-enhanced and 84% for contrast-enhanced sequences) and for T2 at 3 T (69%). DLR demonstrated superior qualitative image quality for all sequences and field strengths (p < 0.001), except for T2 at 3 T, which was observed across all raters (p = 0.670). Diagnostic confidence was similar at 3 T with better but non-significant differences for T2 (p = 0.134) and at 1.5 T with better but non-significant differences for non-contrast-enhanced T1 (p = 0.083) and only marginally significant results for FLAIR (p = 0.033). Both the SSIM and MS-SSIM indicated near-perfect similarity between CR and DLR. FSIM performs worse in terms of consistency between CR and DLR. The image quality metrics NQM, SNR, and PSNR showed better results for DLR. Visual assessment of residual tumor was similar at 3 T but differed at 1.5 T, with more residual tumor detected with DLR, especially by the neurosurgeon (n = 4). Conclusions: An accelerated DLR protocol demonstrates clinical feasibility, enabling high-quality reconstructions in challenging postoperative MRIs. DLR sequences received strong multidisciplinary preference, underscoring their potential to improve neuro-oncologic decision making and suitability for clinical implementation.
Keywords: deep learning; diagnostic accuracy; image reconstruction; intracranial tumors; magnetic resonance imaging; multidisciplinary; postoperative imaging; visual perception preference.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Rykkje A.M., Carlsen J.F., Larsen V.A., Skjøth-Rasmussen J., Christensen I.J., Nielsen M.B., Poulsen H.S., Urup T.H., Hansen A.E. Prognostic relevance of radiological findings on early postoperative MRI for 187 consecutive glioblastoma patients receiving standard therapy. Sci. Rep. 2024;14:10985. doi: 10.1038/s41598-024-61925-3. - DOI - PMC - PubMed
-
- INTERVAL-GB Collaborative. Neurology and Neurosurgery Interest Group (NANSIG) British Neurosurgical Trainee Research Collaborative (BNTRC) Imaging timing after surgery for glioblastoma: An evaluation of practice in Great Britain and Ireland (INTERVAL-GB)—A multi-centre, cohort study. J. Neuro-Oncol. 2024;169:517–529. doi: 10.1007/s11060-024-04705-3. - DOI - PMC - PubMed
-
- Garcia-Ruiz A., Naval-Baudin P., Ligero M., Pons-Escoda A., Bruna J., Plans G., Calvo N., Cos M., Majós C., Perez-Lopez R. Precise enhancement quantification in post-operative MRI as an indicator of residual tumor impact is associated with survival in patients with glioblastoma. Sci. Rep. 2021;11:695. doi: 10.1038/s41598-020-79829-3. - DOI - PMC - PubMed
-
- Kiesel B., Prihoda R., Borkovec M., Mercea P.A., Steindl A., Berghoff A.S., Furtner J., Leitner J., Roetzer T., Preusser M., et al. Postoperative Magnetic Resonance Imaging After Surgery of Brain Metastases: Analysis of Extent of Resection and Potential Risk Factors for Incomplete Resection. World Neurosurg. 2020;143:e365–e373. doi: 10.1016/j.wneu.2020.07.150. - DOI - PubMed
LinkOut - more resources
Full Text Sources

