Investigation of Bacterial Species and Their Antimicrobial Drug Resistance Profile in Feline Urinary Tract Infection in Thailand
- PMID: 40805025
- PMCID: PMC12345554
- DOI: 10.3390/ani15152235
Investigation of Bacterial Species and Their Antimicrobial Drug Resistance Profile in Feline Urinary Tract Infection in Thailand
Abstract
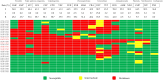
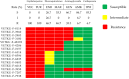
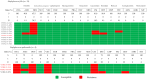
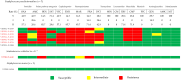
Feline urinary tract infections (UTIs) present a common challenge in veterinary practice, underscoring the importance of understanding local bacterial pathogens and antimicrobial resistance (AMR). This study determined bacterial prevalence and antimicrobial susceptibility in cats at Kasetsart University's Veterinary Teaching Hospital in Bangkok, Thailand. Of the 543 cystocentesis urine samples collected from 428 cats, 115 (21.2%) tested positive for bacterial cultures, leading to a diagnosis of UTIs in 95 cats (22.2%). The most prevalent isolates included Escherichia coli (24.8%), Staphylococcus species (19.2%), Proteus mirabilis (13.6%), Pseudomonas aeruginosa (12.0%), and Enterococcus species (12.0%). Staphylococcus felis (8.8%) and Staphylococcus pseudintermedius (5.6%) were the predominant Staphylococcus species. Rare pathogens such as Corynebacterium urealyticum and Lactococcus garvieae were also identified. Antimicrobial testing revealed alarming resistance, with 69.2% of isolates exhibiting multidrug resistance (MDR). Escherichia coli and Proteus mirabilis showed high resistance to amoxicillin/clavulanic acid (AMC) (45.2-70.6%) and sulfamethoxazole/trimethoprim (SXT) (51.6-52.9%). Enterococcus faecium exhibited 85.7% resistance to AMC. Methicillin resistance was identified in 41.7% of Staphylococcus isolates, particularly high in Staphylococcus epidermidis (75.0%) and Staphylococcus pseudintermedius (71.4%). High fluoroquinolone resistance among MDR isolates further exacerbates AMR concerns. These results indicate that MDR Gram-negative, Staphylococcus, and Enterococcus species complicate the empirical treatment of feline UTIs, highlighting significant implications for AMR in veterinary practice.
Keywords: antimicrobial resistance; bacteria; cat; urinary tract infections.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Antimicrobial susceptibility of bacteria isolated from canine and feline urinary tract samples in New Zealand.N Z Vet J. 2025 Jul 30:1-10. doi: 10.1080/00480169.2025.2533203. Online ahead of print. N Z Vet J. 2025. PMID: 40738173
-
Retrospective analysis of antimicrobial susceptibility testing illustrates the problem of resistant Staphylococcus species in cats in the northeastern United States.Prev Vet Med. 2025 Aug 25;245:106664. doi: 10.1016/j.prevetmed.2025.106664. Online ahead of print. Prev Vet Med. 2025. PMID: 40885083
-
Bacterial profile and antimicrobial susceptibility pattern of urinary tract infection among diabetic patients at Bule Hora University Teaching Hospital, Southern Ethiopia.Sci Rep. 2025 Jul 6;15(1):24112. doi: 10.1038/s41598-025-05782-8. Sci Rep. 2025. PMID: 40619446 Free PMC article.
-
Bacterial profile and antimicrobial resistance patterns of common bacteria among pregnant women with bacteriuria in Ethiopia: a systematic review and meta-analysis.Arch Gynecol Obstet. 2022 Sep;306(3):663-686. doi: 10.1007/s00404-021-06365-4. Epub 2022 Jan 15. Arch Gynecol Obstet. 2022. PMID: 35032208 Free PMC article.
-
Bacterial profile and antimicrobial resistance patterns among pediatrics patients with suspected bloodstream infections in Ethiopia: a systematic review and meta-analysis.BMC Infect Dis. 2025 Aug 9;25(1):1008. doi: 10.1186/s12879-025-11427-y. BMC Infect Dis. 2025. PMID: 40783681 Free PMC article.
References
-
- Vopelius-Feldt C. Ph.D. Thesis. Ludwig-Maximilians-Universität; Munich, Germany: 2012. Bakterielle Harnwegsinfektionen bei der Katze: Eine Retrospektive Uber 10 Jahre.
-
- Gerber B., Boretti F.S., Kley S., Laluha P., Müller C., Sieber N., Unterer S., Wenger M., Flückiger M., Glaus T., et al. Evaluation of clinical signs and causes of lower urinary tract disease in European cats. J. Small Anim. Pract. 2005;46:571–577. doi: 10.1111/j.1748-5827.2005.tb00288.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

