Assessment of Salivary Biomarkers of Gastric Ulcer in Horses from a Clinical Perspective
- PMID: 40805041
- PMCID: PMC12345524
- DOI: 10.3390/ani15152251
Assessment of Salivary Biomarkers of Gastric Ulcer in Horses from a Clinical Perspective
Abstract
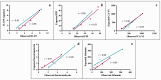
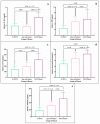
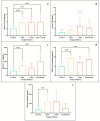
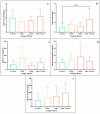
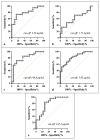
This study arises from the search for non-invasive diagnostic alternatives for equine gastric ulceration (EGUS), which is prevalent, clinically variable and only confirmed by gastroscopy. The aim is to quantify five salivary biomarkers (IL1-F5, PIP, CA VI, serotransferrin, albumin) under clinical conditions by validated assays and analyse their diagnostic value. Horses were grouped in No EGUS (neither clinical signs of EGUS nor gastric lesions), EGUS non-clinical (apparently no clinical signs of EGUS but with gastric lesions), and EGUS clinical (obvious clinical signs of EGUS and with gastric lesions). The concentration of 5 analytes could be quantified using sandwich ELISA assays, with high precision (CV: 6.79-12.38%) and accuracy (>95%). Mean salivary levels of IL1-F5, CA-VI, serotransferrin and albumin were significantly higher in EGUS clinical horses compared to No EGUS horses, whereas PIP showed no statistical significance. EGUS non-clinical horses showed statistical differences with No EGUS horses for PIP and albumin. In addition, IL1-F5, CA-VI, serotransferrin and albumin showed moderate accuracy to distinguish between No EGUS and EGUS clinical horses (AUC ≥ 0.8), with sensitivity and specificity greater than 77% and 65%, respectively. Therefore, these biomarkers could be a promising starting point for screening horse that might have EGUS in practice.
Keywords: diagnostic value; gastric ulceration; horse; markers; quantification; saliva.
Conflict of interest statement
I.R.I. receives consulting fees from Biozyme Incorporated. L.W. was employed by Biozyme Incorporated. These competing interests do not affect the integrity of the study. M.M.-Q. & A.M.G. are researchers who investigated biomarkers that are subject of patent. C.G.-P. declares no conflicts of interest. The results of this study are protected under US patent application USPTO 63/718,379, owned by Biozyme INC.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

