Preliminary Pharmacokinetics and Appetite Stimulant Efficacy of Oral Mirtazapine in Guinea Pigs (Cavia porcellus)
- PMID: 40805046
- PMCID: PMC12345486
- DOI: 10.3390/ani15152256
Preliminary Pharmacokinetics and Appetite Stimulant Efficacy of Oral Mirtazapine in Guinea Pigs (Cavia porcellus)
Abstract
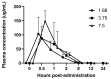
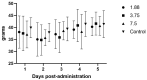
Guinea pigs used in research may experience inappetence or decreased intestinal motility, which can significantly compromise their welfare. This study evaluates the use of mirtazapine on appetite and intestinal motility in guinea pigs. An initial pharmacokinetics and efficacy study was performed using healthy male guinea pigs administered mirtazapine at 1.88, 3.75, or 7.5 mg orally once daily for four days (n = 6), in a crossover design where all animals received all doses. Body, feed, and fecal weights were taken daily for 4 days. There were no significant differences in weight gains, feed intake, or fecal output as compared to guinea pigs given saline only (n = 3). Blood was collected under anesthesia at 0, 0.5, 1, 2, 8, 12, and 24 h post-administration. Pharmacokinetic analysis completed after the first dose showed peak plasma levels at 30 min, then falling below the limit of detection between 8 h and 12 h at all doses. Based on the pharmacokinetic profile, a follow-up study was performed in another set of healthy male guinea pigs with every 8 h dosing at 1.88 mg orally for 5 days (n = 6). There was a significant increase in feed intake during mirtazapine administration as compared to baseline intake, but no significant difference in weight gains. This study shows that mirtazapine can be used as an appetite stimulant in guinea pigs but must be dosed at least every eight hours to be effective.
Keywords: anorexia; appetite; guinea pig; inappetence; mirtazapine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Orme I.M. Mouse and guinea pig models for testing new tuberculosis vaccines. Tuberc. Edinb. Scotl. 2005;85:13–17. - PubMed
-
- Chiou P.W.S., Yu B., Kuo C.Y. Comparison of digestive function among rabbits, guinea-pigs, rats and hamsters. I. Performance, digestibility and rate of digesta passage. Asian-Australas. J. Anim. Sci. 2000;13:1499–1507. doi: 10.5713/ajas.2000.1499. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

