Tea Polyphenols Mitigate TBBPA-Induced Renal Injury Through Modulation of ROS-PI3K/AKT-NF-κB Signalling in Carp (Cyprinus carpio)
- PMID: 40805095
- PMCID: PMC12345511
- DOI: 10.3390/ani15152307
Tea Polyphenols Mitigate TBBPA-Induced Renal Injury Through Modulation of ROS-PI3K/AKT-NF-κB Signalling in Carp (Cyprinus carpio)
Abstract
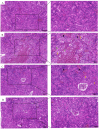
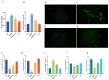
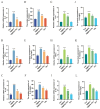
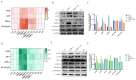
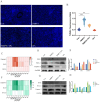
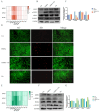
Tetrabromobisphenol A (TBBPA), a widely utilised brominated flame retardant, demonstrates toxicological effects in aquatic organisms. Tea polyphenols (TPs), natural compounds found in tea leaves, exhibit both antioxidant and anti-inflammatory activities. The kidney is one of the major metabolic organs in common carp and serves as a target organ for toxic substances. This study evaluated the therapeutic potential of TPs in mitigating TBBPA-induced nephrotoxicity in common carp. Common carp were exposed to 0.5 mg/L TBBPA in water and/or fed a diet supplemented with 1 g/kg TPs for 14 days. In vitro, primary renal cells were treated with 60 μM TBBPA and/or 2.5 μg/L TPs for 24 h. Methods included histopathology, TUNEL assay for apoptosis, ROS detection, and molecular analyses. Antioxidant enzymes (SOD, CAT) and inflammatory cytokines (IL-1β, IL-6, TNF-α) were quantified using ELISA kits. Results showed that TBBPA induced oxidative stress, and activated the ROS-PI3K/AKT-NF-κB pathway, thereby resulting in inflammatory responses. TBBPA upregulated apoptosis-related genes (Caspase-3, Bax, and Bcl-2) and induced apoptosis. TBBPA upregulated the expression of RIPK3/MLKL, thereby exacerbating necroptosis. TPs intervention significantly mitigated these effects by reducing ROS, suppressing NF-κB activation, and restoring antioxidant enzyme activities (SOD, CAT). Moreover, TPs attenuated apoptosis and necrosis in the carp kidney, thereby enhancing the survival ability and immunity of common carp.
Keywords: aquaculture feed additives; inflammatory injury; oxidative stress; tea polyphenols.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Microbial dysbiosis induced in Cyprinus carpio by tetrabromobisphenol A exposure: Mediation through gut barrier impairment and oxidative stress.Comp Biochem Physiol Part D Genomics Proteomics. 2025 Aug 19;56:101609. doi: 10.1016/j.cbd.2025.101609. Online ahead of print. Comp Biochem Physiol Part D Genomics Proteomics. 2025. PMID: 40845582
-
Dietary Tea Polyphenols Improve Growth Performance and Intestinal Microbiota Under Chronic Crowding Stress in Hybrid Crucian Carp.Animals (Basel). 2025 Jul 5;15(13):1983. doi: 10.3390/ani15131983. Animals (Basel). 2025. PMID: 40646882 Free PMC article.
-
Oxidative Stress, Energy Metabolism Disorder, Mitochondrial Damage, and miR-144 Participated in Molecular Mechanisms of 4-Octylphenol-Caused Cardiac Autophagic Damage in Common Carps (Cyprinus carpio L.).Metabolites. 2025 Jun 11;15(6):391. doi: 10.3390/metabo15060391. Metabolites. 2025. PMID: 40559415 Free PMC article.
-
Buzhong Yiqi decoction improves inflammation and oxidative damage in autoimmune thyroiditis by inhibiting apoptosis via the SIRT1-Mediated Nrf2/NF-κB axis.J Ethnopharmacol. 2025 Jul 24;351:119967. doi: 10.1016/j.jep.2025.119967. Epub 2025 May 11. J Ethnopharmacol. 2025. PMID: 40360040
-
Remote Ischemic Postconditioning Improve Cerebral Ischemia-Reperfusion Injury Induced Cognitive Dysfunction through Suppressing Mitochondrial Apoptosis in Hippocampus via TK/BK/B2R-Mediated PI3K/AKT.Mol Neurobiol. 2025 Aug;62(8):10652-10669. doi: 10.1007/s12035-025-04864-y. Epub 2025 Apr 14. Mol Neurobiol. 2025. PMID: 40229456 Free PMC article.
References
-
- Song Y., Zhang W. Balancing Growth and Sustainability in China’s Carp Aquaculture: Practices, Policies, and Sustainability Pathways. Sustainability. 2025;17:5593. doi: 10.3390/su17125593. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

