The HIV Protease Inhibitor Ritonavir Reverts the Mesenchymal Phenotype Induced by Inflammatory Cytokines in Normal and Tumor Oral Keratinocytes to an Epithelial One, Increasing the Radiosensitivity of Tumor Oral Keratinocytes
- PMID: 40805215
- PMCID: PMC12346649
- DOI: 10.3390/cancers17152519
The HIV Protease Inhibitor Ritonavir Reverts the Mesenchymal Phenotype Induced by Inflammatory Cytokines in Normal and Tumor Oral Keratinocytes to an Epithelial One, Increasing the Radiosensitivity of Tumor Oral Keratinocytes
Abstract
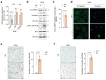
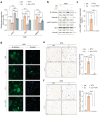
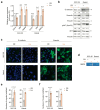
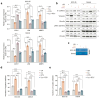
Background/Objectives: During the repair of a wounded epithelium, keratinocytes become invasive via the epithelial-to-mesenchymal transition (EMT) process. Usually temporary and controlled, EMT persists in a chronically inflamed epithelium and is exacerbated in epithelial dysplasia and dysregulated in invasive carcinomas. Here we investigated the effects that IL-1 beta, IL-6, and IL-8, inflammatory cytokines expressed in specimens from OPMDs and OSCCs, have on NOKs and OSCC cells. Methods: AKT activation and EMT induction were assessed along with cellular invasiveness. Results: IL-1 beta, IL-6, and IL-8 induced EMT in NOKs, ex novo conferring them invasive capacity. The same cytokines exacerbated the constitutive EMT and invasiveness of OSCC cells. Since these phenomena were accompanied by AKT activation, we tested whether they could be influenced by RTV, a long-used anti-HIV drug that was previously found to block the activation of human AKT and exert antitumor effects. We observed that therapeutic amounts of RTV counteract all the above-mentioned tumorigenic activities of ILs. Finally, consistent with the key role that AKT and EMT play in OSCC radio-resistance, RTV increased OSCC cells' sensitivity to therapeutic doses of ionizing radiation. Conclusions: These preliminary in vitro findings encourage the use of RTV to prevent the malignant evolution of OPMDs, reduce the risk of OSCC metastasis, and improve the outcomes of anti-OSCC radiotherapy.
Keywords: AKT; EMT; OPMD; OSCC; cell invasion; cell survival; inflammation; interleukins; radiosensitivity; ritonavir.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Chondroitin sulfate proteoglycan 4 increases invasion of recessive dystrophic epidermolysis bullosa-associated cutaneous squamous cell carcinoma by modifying transforming growth factor-β signalling.Br J Dermatol. 2024 Dec 23;192(1):104-117. doi: 10.1093/bjd/ljae295. Br J Dermatol. 2024. PMID: 39018437 Free PMC article.
-
KSHV and HPV modulate epithelial-to-mesenchymal transition in oral epithelial cells.mBio. 2025 Aug 15:e0048425. doi: 10.1128/mbio.00484-25. Online ahead of print. mBio. 2025. PMID: 40815147
-
Clinical assessment for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults.Cochrane Database Syst Rev. 2021 Dec 10;12(12):CD010173. doi: 10.1002/14651858.CD010173.pub3. Cochrane Database Syst Rev. 2021. PMID: 34891214 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Pomella S., Melaiu O., Dri M., Martelli M., Gargari M., Barillari G. Effects of Angiogenic Factors on the Epithelial-to-Mesenchymal Transition and Their Impact on the Onset and Progression of Oral Squamous Cell Carcinoma: An Overview. Cells. 2024;13:1294. doi: 10.3390/cells13151294. - DOI - PMC - PubMed
-
- Masola V., Carraro A., Granata S., Signorini L., Bellin G., Violi P., Lupo A., Tedeschi U., Onisto M., Gambaro G., et al. In Vitro Effects of Interleukin (IL)-1 Beta Inhibition on the Epithelial-to-Mesenchymal Transition (EMT) of Renal Tubular and Hepatic Stellate Cells. J. Transl. Med. 2019;17:12. doi: 10.1186/s12967-019-1770-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

