Integrating Circulating Tumor DNA into Clinical Management of Colorectal Cancer: Practical Implications and Therapeutic Challenges
- PMID: 40805216
- PMCID: PMC12346868
- DOI: 10.3390/cancers17152520
Integrating Circulating Tumor DNA into Clinical Management of Colorectal Cancer: Practical Implications and Therapeutic Challenges
Abstract
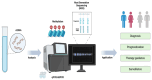
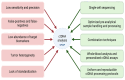
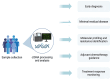
The American Cancer Society estimates that over 152,000 new cases of colorectal cancer (CRC) were diagnosed in 2024, with more than 105,000 cases affecting the colon and 46,000 involving the rectum. CRC remains the second leading cause of cancer-related deaths in the United States, with an estimated 53,010 deaths in 2024. In the era of precision medicine, which incorporates molecular and environmental information into clinical decision-making, identifying patients harboring a deficiency in Deoxyribonucleic acid (DNA) repair allowed for targeted immunotherapies and significantly reduced CRC-related mortality. A significant advancement in this domain is the application of liquid biopsy, which has emerged as a promising tool for prognostication, guiding therapy, and monitoring treatment response in CRC. This review aims to comprehensively explore the role of liquid biopsy in colorectal malignancies, describing its practical applications, prognostic significance, and potential to revolutionize CRC management in the future. At the end, we also aim to show a schematic representation of showing integration of Circulating Tumor (Ct) DNA in routine clinical management of CRC. The highlight of this article is the structured and evidence-based schematic framework and its integration into future practice. The schematic pathway is designed to optimize ctDNA utilization across various stages of colorectal cancer management.
Keywords: colorectal cancer; ctDNA; prognosis; surveillance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Recent advances in liquid biopsy for precision oncology: emerging biomarkers and clinical applications in lung cancer.Future Oncol. 2025 Aug 5:1-19. doi: 10.1080/14796694.2025.2542051. Online ahead of print. Future Oncol. 2025. PMID: 40762271 Review.
-
Guaiac-based faecal occult blood tests versus faecal immunochemical tests for colorectal cancer screening in average-risk individuals.Cochrane Database Syst Rev. 2022 Jun 6;6(6):CD009276. doi: 10.1002/14651858.CD009276.pub2. Cochrane Database Syst Rev. 2022. PMID: 35665911 Free PMC article.
-
Circulating-tumour DNA methylation of HAND1 gene: a promising biomarker in early detection of colorectal cancer.BMC Med Genomics. 2024 Apr 30;17(1):117. doi: 10.1186/s12920-024-01893-9. BMC Med Genomics. 2024. PMID: 38689296 Free PMC article.
References
-
- Van Cutsem E., Peeters M., Siena S., Humblet Y., Hendlisz A., Neyns B., Canon J.L., Van Laetham J.L., Maurel J., Richardson G., et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

