Efficacy of NAMPT Inhibitors in Pancreatic Cancer After Stratification by MAP17 (PDZK1IP1) Levels
- PMID: 40805270
- PMCID: PMC12346621
- DOI: 10.3390/cancers17152575
Efficacy of NAMPT Inhibitors in Pancreatic Cancer After Stratification by MAP17 (PDZK1IP1) Levels
Abstract
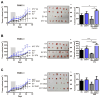
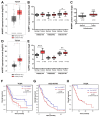
Background/Objectives: Pancreatic cancer (PC) is the seventh leading cause of cancer-related deaths worldwide, with its incidence rising each year. Despite its relatively low incidence, the aggressiveness of pancreatic cancer results in high mortality, with only 12% of patients surviving five years post-diagnosis. Surgical resection remains the only potentially curative treatment, but the tumor is often diagnosed at an advanced stage. The goal of this work is to identify vulnerabilities that can affect the efficacy of treatments and improve the efficacy of therapy. Methods: MAP17 overexpression in pancreatic cancer cell lines, RT-qPCR analysis, xenografts, in vitro and in vivo treatments, analysis of data from pancreatic tumors in transcriptomic patient databases. Results: We studied the prognostic and predictive value of MAP17 (PDZK1IP1) expression in pancreatic cancer, and we found that high MAP17 mRNA expression was associated with poor prognosis. In addition, single-cell analysis revealed that high MAP17 expression was present only in tumor cells. We investigated whether the response to various antitumor agents depended on MAP17 expression. In 2D culture, MAP17-expressing pancreatic cancer cells responded better to gemcitabine and 5-fluorouracil. However, in vivo xenograft tumors with MAP17 expression showed resistance to all treatments. Additionally, MAP17-expressing cells had a high NAD pool, which seems to be effectively depleted in vivo by NAMPT inhibitors, the primary enzyme for NAD biosynthesis. Conclusions: Our findings suggest that MAP17 expression could enhance the prognostic stratification of pancreatic cancer patients. Moreover, the coadministration of NAMPT inhibitors with current treatments may sensitize tumors with high MAP17 expression to chemotherapy and improve the efficacy of chemotherapy.
Keywords: MAP17; NAD; biomarkers; chemotherapy; pancreatic cancer; prognosis.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures




References
-
- Partyka O., Pajewska M., Kwaśniewska D., Czerw A., Deptała A., Budzik M., Cipora E., Gąska I., Gazdowicz L., Mielnik A., et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers. 2023;15:3634. doi: 10.3390/cancers15143634. - DOI - PMC - PubMed
-
- Conroy T., Pfeiffer P., Vilgrain V., Lamarca A., Seufferlein T., O’rEilly E., Hackert T., Golan T., Prager G., Haustermans K., et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023;34:987–1002. doi: 10.1016/j.annonc.2023.08.009. - DOI - PubMed
-
- Latenstein A.E., van der Geest L.G., Bonsing B.A., Koerkamp B.G., Mohammad N.H., de Hingh I.H., de Meijer V.E., Molenaar I.Q., van Santvoort H.C., van Tienhoven G., et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur. J. Cancer. 2020;125:83–93. doi: 10.1016/j.ejca.2019.11.002. - DOI - PubMed
Grants and funding
- RTI2018-097455-B-I00/Ministerio de Ciencia, Innovación y Universidades
- PID2021-122629OB-I00/Ministerio de Ciencia, Innovación y Universidades
- RED2018-102723-T/Ministerio de Ciencia, Innovación y Universidades
- CB16/12/00275/CIBER de Cáncer
- PI-0397-2017/Consejería de Salud (Junta de Andalucía)
- P18-RT-2501/Consejería de Economía, Conocimiento, Empresas y Universidad de la Junta de Andalucía
- GC16173720CARR/AECC Foundation (Spanish Association of Cancer Research)
- CTEICU/PAIDI 2020, DOC_01655/Consejería de Transformación Económica, Industria, Conocimiento, y Universidades de la Junta de Andalucía
- ISCIII, CM18/00219/Instituto de Salud Carlos III
LinkOut - more resources
Full Text Sources
Miscellaneous

