Long-Term Culture of Cellular Spheroids in Novel Hydrogel Constructs for ECM Characterization in Bone Models
- PMID: 40805416
- PMCID: PMC12347893
- DOI: 10.3390/ma18153538
Long-Term Culture of Cellular Spheroids in Novel Hydrogel Constructs for ECM Characterization in Bone Models
Abstract
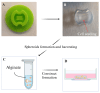
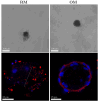
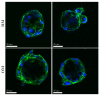
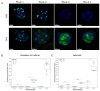
The application of cellular spheroids in bone tissue engineering research has gained significant interest in the last decade. Compared to monolayer cell cultures, the 3D architecture allows for more physiological cell-cell and cell-extracellular matrix (ECM) interactions that make cellular spheroids a suitable model system to investigate the bone ECM in vitro. The use of 3D model systems requires fine-tuning of the experimental methods used to study cell morphology, ECM deposition and mineralization, and cell-ECM interactions. In this study, we use a construct made of MC3T3-E1 cellular spheroids encapsulated in an alginate hydrogel to study and characterize the deposited ECM. Spheroid shape and structure were evaluated using confocal microscopy. The deposited collagenous ECM was characterized using Second Harmonic Imaging Microscopy (SHIM), quantitative hydroxyproline (HYP) assay, and Transmission Electron Microscopy (TEM). The use of hydrogel constructs enabled easy handling and imaging of the samples, while also helping to preserve the spheroid's stability by preventing cells from adhering to the culture dish surface. We used a non-modified alginate hydrogel that did not facilitate cell attachment and therefore functioned as an inert encapsulating scaffold. Constructs were cultured for up to 4 weeks. SHIM, HYP assay, and TEM confirmed the deposition of a collagenous matrix. We demonstrated that alginate-encapsulated bone spheroids are a convenient and promising model for studying the bone ECM in vitro.
Keywords: SHIM; TEM; collagen; immunofluorescence; spheroids.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
LinkOut - more resources
Full Text Sources

