Synthesis and FT-IR/Raman Characterization of a Graphene Oxide-Methacrylamide Monomer for Dental Applications
- PMID: 40805429
- PMCID: PMC12347912
- DOI: 10.3390/ma18153550
Synthesis and FT-IR/Raman Characterization of a Graphene Oxide-Methacrylamide Monomer for Dental Applications
Abstract
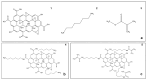
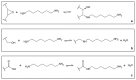
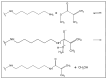
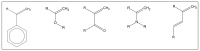
Background: Graphene oxide (GO) is widely explored as a functional additive in polymer composites; however, its simple physical dispersion in dental resins often leads to poor interfacial stability and limited long-term performance. Covalent functionalization may overcome these limitations by enabling chemical integration into the polymer matrix. This study presents the synthesis and FT-IR/Raman characterization of GRAPHYMERE®, a novel graphene oxide-based monomer obtained through exfoliation, amine functionalization with 1,6-hexanediamine, and transamidation with methyl methacrylate.
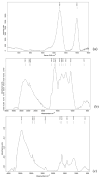
Methods: A novel GO-based monomer, GRAPHYMERE®, was synthesized through a three-step process involving GO exfoliation, amine functionalization with 1,6-hexanediamine, and transamidation with methyl methacrylate to introduce polymerizable acrylic groups. The resulting product was characterized using FT-IR and Raman spectroscopy.
Results: Spectroscopic analyses confirmed the presence of aliphatic chains and amine functionalities on the GO surface. Although some expected signals were overlapped, the data suggest successful surface modification and partial insertion of methacrylamide groups. The process is straightforward, uses low-toxicity reagents, and avoids complex reaction steps.
Conclusions: GRAPHYMERE® represents a chemically modified GO monomer potentially suitable for copolymerization within dental resin matrices. While its structural features support compatibility with radical polymerization systems, further studies are required to assess its mechanical performance and functional properties in dental resin applications.
Keywords: GRAPHYMERE; composite; dental materials; graphene; graphene oxide; polymer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Elbow Fractures Overview.2025 Jul 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28723005 Free Books & Documents.
-
Direct composite resin fillings versus amalgam fillings for permanent posterior teeth.Cochrane Database Syst Rev. 2021 Aug 13;8(8):CD005620. doi: 10.1002/14651858.CD005620.pub3. Cochrane Database Syst Rev. 2021. PMID: 34387873 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Atraumatic restorative treatment versus conventional restorative treatment for managing dental caries.Cochrane Database Syst Rev. 2017 Dec 28;12(12):CD008072. doi: 10.1002/14651858.CD008072.pub2. Cochrane Database Syst Rev. 2017. PMID: 29284075 Free PMC article.
References
-
- Grodzinski J. Biomedical application of functional polymers. React. Funct. Polym. 1999;39:99–138. doi: 10.1016/S1381-5148(98)00054-6. - DOI
-
- Patil A., Patel A., Purohit R. An overview of polymeric materials for automotive applications. Mater. Today Proc. 2017;4:3807–3815. doi: 10.1016/j.matpr.2017.02.278. - DOI
-
- Bashir M., Rajendran P. A review on electroactive polymers development for aerospace applications. J. Intell. Mater. Syst. Struct. 2018;29:3681–3695. doi: 10.1177/1045389X18798951. - DOI
-
- Kumar A., Srivastava A., Galaev I.Y., Mattiasson B. Smart polymers: Physical forms and bioengineering applications. Prog. Polym. Sci. 2007;32:1205–1237. doi: 10.1016/j.progpolymsci.2007.05.003. - DOI
-
- Das T.K., Prusty S. Review on conducting polymers and their applications. Polym. Plast. Technol. Eng. 2012;51:1487–1500. doi: 10.1080/03602559.2012.710697. - DOI
LinkOut - more resources
Full Text Sources

