Unlocking the Potential of Gracilaria chilensis Against Prostate Cancer
- PMID: 40805701
- PMCID: PMC12349654
- DOI: 10.3390/plants14152352
Unlocking the Potential of Gracilaria chilensis Against Prostate Cancer
Abstract
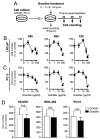
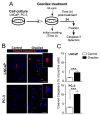
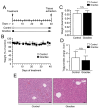
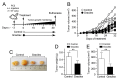
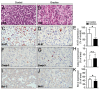
Prostate cancer (PCa) is the second leading cause of cancer-related death among men in most Western countries. Current therapies for PCa are limited, often ineffective, and associated with significant side effects. As a result, there is a growing interest in exploring new therapeutic agents, particularly from the polyphyletic group of algae, which offers a promising source of compounds with anticancer properties. Our research group has focused on investigating the effects of a novel oleoresin from Gracilaria chilensis, known as Gracilex®, as a potential therapeutic agent against PCa using both in vitro and in vivo models. Our findings indicate that Gracilex® exhibits a time- and dose-dependent inhibitory effect on cell survival in LNCaP and PC-3 PCa, reducing viability by over 50% and inducing apoptosis, as evidenced by a significant increase in activated caspase-3 expression in both cell lines. Moreover, Gracilex® significantly reduces the proliferation rate of both LNCaP and PC-3 prostate cancer cell lines, as evidenced by a marked decrease in the growth curve slope (p = 0.0034 for LNCaP; p < 0.0001 for PC-3) and a 40-50% reduction in the proportion of Ki-67-positive PCa cells. In addition, Gracilex® significantly reduces in vitro cell migration and invasion in LNCaP and PC-3 cell lines. Lastly, Gracilex® inhibits tumor growth in an in vivo xenograft model, an effect that correlates with the reduced PCa cell proliferation observed in tumor tissue sections. Collectively, our data strongly support the broad antitumoral effects of Gracilex® on PCa cells in vitro and in vivo. These findings advance our understanding of its potential therapeutic role in PCa and highlight the relevance of further investigating algae-derived compounds for cancer treatment.
Keywords: Gracilex®; cancer; oleoresin; prostate cancer; seaweed.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

