An Improved Agrobacterium-Mediated Transformation Method for an Important Fresh Fruit: Kiwifruit (Actinidia deliciosa)
- PMID: 40805702
- PMCID: PMC12349596
- DOI: 10.3390/plants14152353
An Improved Agrobacterium-Mediated Transformation Method for an Important Fresh Fruit: Kiwifruit (Actinidia deliciosa)
Abstract
Genetic transformation is an essential tool for investigating gene function and editing genomes. Kiwifruit, recognized as a significant global fresh fruit crop, holds considerable economic and nutritional importance. However, current genetic transformation techniques for kiwifruit are impeded by low efficiency, lengthy culture durations (a minimum of six months), and substantial labor requirements. In this research, we established an efficient system for shoot regeneration and the stable genetic transformation of the 'Hayward' cultivar, utilizing leaf explants in conjunction with two strains of Agrobacterium that harbor the expression vector pBI121-35S::GFP, which contains the green fluorescent protein (GFP) gene as a visible marker within the T-DNA region. Our results show that 93.3% of leaf explants responded positively to the regeneration medium, producing multiple independent adventitious shoots around the explants within a six-week period. Furthermore, over 71% of kanamycin-resistant plantlets exhibited robust GFP expression, and the entire transformation process was completed within four months of culture. Southern blot analysis confirmed the stable integration of GFP into the genome, while RT-PCR and fluorescence microscopy validated the sustained expression of GFP in mature plants. This efficient protocol for regeneration and transformation provides a solid foundation for micropropagation and the enhancement of desirable traits in kiwifruit through overexpression and gene silencing techniques.
Keywords: Southern blot analysis; high-efficiency genetic transformation; kiwifruit (Actinidia deliciosa); leaf explant; mature plant; stable GFP expression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

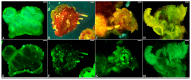

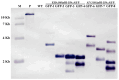


References
-
- McGhie T.K. Secondary metabolite components of kiwifruit. Adv. Food Nutr. Res. 2013;68:101–124. - PubMed
-
- Tavarini S., Degl’iNnocenti E., Remorini D., Massai R., Guidi L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008;107:282–288. doi: 10.1016/j.foodchem.2007.08.015. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

