Unveiling Stage-Specific Flavonoid Dynamics Underlying Drought Tolerance in Sweet Potato (Ipomoea batatas L.) via Integrative Transcriptomic and Metabolomic Analyses
- PMID: 40805732
- PMCID: PMC12349511
- DOI: 10.3390/plants14152383
Unveiling Stage-Specific Flavonoid Dynamics Underlying Drought Tolerance in Sweet Potato (Ipomoea batatas L.) via Integrative Transcriptomic and Metabolomic Analyses
Abstract
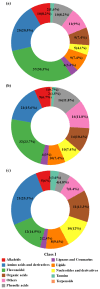
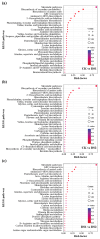
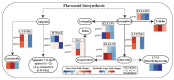
Drought stress severely limits the productivity of sweet potato (Ipomoea batatas L.), yet the stage-specific molecular mechanisms of its adaptation remain poorly understood. Therefore, we integrated transcriptomics and extensive targeted metabolomics analysis to investigate the drought responses of the sweet potato cultivar 'Luoyu 11' during the branching and tuber formation stage (DS1) and the storage root expansion stage (DS2) under controlled drought conditions (45 ± 5% field capacity). Transcriptome analysis identified 8292 and 13,509 differentially expressed genes in DS1 and DS2, respectively, compared with the well-watered control (75 ± 5% field capacity). KEGG enrichment analysis revealed the activation of plant hormone signaling, carbon metabolism, and flavonoid biosynthesis pathways, and more pronounced transcriptional changes were observed during the DS2 stage. Metabolomic analysis identified 415 differentially accumulated metabolites across the two growth periods, with flavonoids being the most abundant (accounting for 30.3% in DS1 and 23.7% in DS2), followed by amino acids and organic acids, which highlighted their roles in osmotic regulation and oxidative stress alleviation. Integrated omics analysis revealed stage-specific regulation of flavonoid biosynthesis under drought stress. Genes such as CYP75B1 and IF7MAT were consistently downregulated, whereas flavonol synthase and glycosyltransferases exhibited differential expression patterns, which correlated with the selective accumulation of trifolin and luteoloside. Our findings provide novel insights into the molecular basis of drought tolerance in sweet potato and offer actionable targets for breeding and precision water management in drought-prone regions.
Keywords: drought stress; flavonoid biosynthesis; metabolomics; stage-specific adaptation; transcriptomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Integrated Transcriptome and Metabolome Analysis Provides Insights into the Low-Temperature Response in Sweet Potato (Ipomoea batatas L.).Genes (Basel). 2025 Jul 28;16(8):899. doi: 10.3390/genes16080899. Genes (Basel). 2025. PMID: 40869948 Free PMC article.
-
Transcriptomic and metabolomic analyses of root responses in Indigofera stachyodes seedlings under drought stress: a medicinal plant native to karst mountainous regions.Front Plant Sci. 2025 Jul 1;16:1607789. doi: 10.3389/fpls.2025.1607789. eCollection 2025. Front Plant Sci. 2025. PMID: 40666302 Free PMC article.
-
Genome-wide identification of GATA family genes in sweet potato (Ipomoea batatas L.) and their expression patterns under abiotic stress.Front Genet. 2025 Jul 9;16:1635749. doi: 10.3389/fgene.2025.1635749. eCollection 2025. Front Genet. 2025. PMID: 40772271 Free PMC article.
-
Sweet potato for type 2 diabetes mellitus.Cochrane Database Syst Rev. 2013 Sep 3;2013(9):CD009128. doi: 10.1002/14651858.CD009128.pub3. Cochrane Database Syst Rev. 2013. PMID: 24000051 Free PMC article.
-
Sweet potato for type 2 diabetes mellitus.Cochrane Database Syst Rev. 2012 Feb 15;(2):CD009128. doi: 10.1002/14651858.CD009128.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2013 Sep 03;(9):CD009128. doi: 10.1002/14651858.CD009128.pub3. PMID: 22336861 Updated.
References
-
- Motsa M.M., Modi A.T., Mabhaudhi T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. S. Afr. J. Sci. 2015;111:1–8. doi: 10.17159/sajs.2015/20140252. - DOI
-
- Gajanayake B., Reddy K.R., Shankle M.W., Arancibia R.A. Early-season soil moisture deficit reduces sweetpotato storage root initiation and development. HortScience. 2013;48:1457–1462. doi: 10.21273/HORTSCI.48.12.1457. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

