Raising pH Reduces Manganese Toxicity in Citrus grandis (L.) Osbeck by Efficient Maintenance of Nutrient Homeostasis to Enhance Photosynthesis and Growth
- PMID: 40805740
- PMCID: PMC12349334
- DOI: 10.3390/plants14152390
Raising pH Reduces Manganese Toxicity in Citrus grandis (L.) Osbeck by Efficient Maintenance of Nutrient Homeostasis to Enhance Photosynthesis and Growth
Abstract
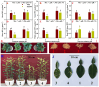
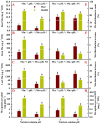
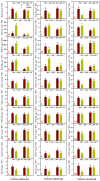
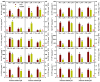
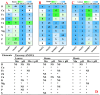
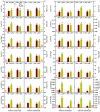
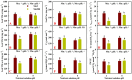
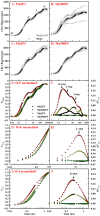
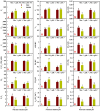
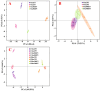
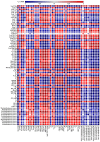
Manganese (Mn) excess and low pH often coexist in some citrus orchard soils. Little information is known about the underlying mechanism by which raising pH reduces Mn toxicity in citrus plants. 'Sour pummelo' (Citrus grandis (L.) Osbeck) seedlings were treated with 2 (Mn2) or 500 (Mn500) μM Mn at a pH of 3 (P3) or 5 (P5) for 25 weeks. Raising pH mitigated Mn500-induced increases in Mn, iron, copper, and zinc concentrations in roots, stems, and leaves, as well as nitrogen, phosphorus, potassium, calcium, magnesium, sulfur, copper, iron, and zinc distributions in roots, but it mitigated Mn500-induced decreases in nitrogen, phosphorus, potassium, calcium, magnesium, sulfur, and boron concentrations in roots, stems, and leaves, as well as nutrient imbalance. Raising pH mitigated Mn500-induced necrotic spots on old leaves, yellowing of young leaves, decreases in seedling growth, leaf chlorophyll concentration, and CO2 assimilation (ACO2), increase in root dry weight (DW)/shoot DW, and alterations of leaf chlorophyll a fluorescence (OJIP) transients and related indexes. Further analysis indicated that raising pH ameliorated Mn500-induced impairment of nutrient homeostasis, leaf thylakoid structure by iron deficiency and competition of Mn with magnesium, and photosynthetic electron transport chain (PETC), thereby reducing Mn500-induced declines in ACO2 and subsequent seedling growth. These results validated the hypothesis that raising pH reduced Mn toxicity in 'Sour pummelo' seedlings by (a) reducing Mn uptake, (b) efficient maintenance of nutrient homeostasis under Mn stress, (c) reducing Mn excess-induced impairment of thylakoid structure and PEPC and inhibition of chlorophyll biosynthesis, and (d) increasing ACO2 and subsequent seedling growth under Mn excess.
Keywords: CO2 assimilation; OJIP transient; manganese excess; nutrient homeostasis; thylakoid structure.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Broadley M., Brown P., Cakmak I., Rengel Z., Zhao F. Function of nutrients: Micronutrients. In: Marschner P., editor. Marschner’s Mineral Nutrition of Higher Plants. 3rd ed. Academic; Amsterdam, The Netherlands: 2012. pp. 191–248.
-
- Zheng Z.-C., Chen H.-H., Yang H., Shen Q., Chen X.-F., Huang W.-L., Yang L.-T., Guo J., Chen L.-S. Citrus sinensis manganese tolerance: Insight from manganese-stimulated secretion of root exudates and rhizosphere alkalization. Plant Physiol. Biochem. 2024;206:108318. doi: 10.1016/j.plaphy.2023.108318. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

