HPLC-ESI-HRMS/MS-Based Metabolite Profiling and Bioactivity Assessment of Catharanthus roseus
- PMID: 40805744
- PMCID: PMC12348932
- DOI: 10.3390/plants14152395
HPLC-ESI-HRMS/MS-Based Metabolite Profiling and Bioactivity Assessment of Catharanthus roseus
Abstract
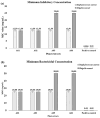
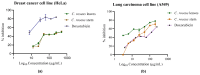
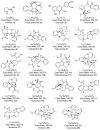
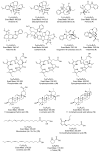
A comprehensive metabolic profiling of Catharanthus roseus (L.) G. Don was performed using tandem mass spectrometry, along with an evaluation of the biological activities of its various solvent extracts. Among these, the methanolic leaf extract exhibited mild radical scavenging activity, low to moderate antimicrobial activity, and limited cytotoxicity in both the brine shrimp lethality assay and MTT assay against HeLa and A549 cell lines. High-performance liquid chromatography-electrospray ionization-high-resolution tandem mass spectrometry (HPLC-ESI-HRMS/MS) analysis led to the annotation of 34 metabolites, primarily alkaloids. These included 23 indole alkaloids, two fatty acids, two pentacyclic triterpenoids, one amino acid, four porphyrin derivatives, one glyceride, and one chlorin derivative. Notably, two metabolites-2,3-dihydroxypropyl 9,12,15-octadecatrienoate and (10S)-hydroxypheophorbide A-were identified for the first time in C. roseus. Furthermore, Global Natural Products Social Molecular Networking (GNPS) analysis revealed 18 additional metabolites, including epoxypheophorbide A, 11,12-dehydroursolic acid lactone, and 20-isocatharanthine. These findings highlight the diverse secondary metabolite profile of C. roseus and support its potential as a source of bioactive compounds for therapeutic development.
Keywords: Catharanthus roseus; GNPS; antimicrobial assay; radical scavenging activity; secondary metabolites.
Conflict of interest statement
Author Salyan Bhattarai was employed by the company Paraza Pharma, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Castiglioni A. A History of Medicine. Routledge; London, UK: 2019.
-
- Parsaeimehr A., Martinez-Chapa S., Parra-Saldívar R. The Microbiology of Skin, Soft Tissue, Bone and Joint Infections. Elsevier; Amsterdam, The Netherlands: 2017. Medicinal plants versus skin disorders: A survey from ancient to modern herbalism; pp. 205–221.
-
- Rakotoarivelo N.H., Rakotoarivony F., Ramarosandratana A.V., Jeannoda V.H., Kuhlman A.R., Randrianasolo A., Bussmann R.W. Medicinal plants used to treat the most frequent diseases encountered in Ambalabe rural community, Eastern Madagascar. J. Ethnobiol. Ethnomed. 2015;11:68. doi: 10.1186/s13002-015-0050-2. - DOI - PMC - PubMed
-
- Tiwari B., Raut B. Screening of selected medicinal plants of Pokhara valley for their antimicrobial activities. J. Nat. Hist. Mus. 2009;24:16–20. doi: 10.3126/jnhm.v24i1.2232. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

