Identification and Characterization of NAC Transcription Factors Involved in Pine Wilt Nematode Resistance in Pinus massoniana
- PMID: 40805748
- PMCID: PMC12349346
- DOI: 10.3390/plants14152399
Identification and Characterization of NAC Transcription Factors Involved in Pine Wilt Nematode Resistance in Pinus massoniana
Abstract
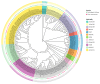
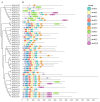
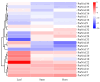
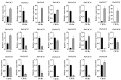
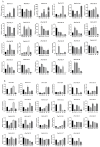
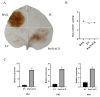
Pinus massoniana Lamb. is an economically important conifer native to China. However, it is highly susceptible to the pine wood nematode (Bursaphelenchus xylophilus, PWN), the causal agent of pine wilt disease (PWD), resulting in substantial ecological and economic losses. To elucidate potential molecular defense mechanisms, 50 NAC (NAM, ATAF1/2, and CUC2) transcription factors (PmNACs) were identified in the P. massoniana genome. Phylogenetic analysis divided these PmNACs into seven subfamilies, and motif analysis identified ten conserved motifs associated with stress responses. Twenty-three genes were selected for expression analysis in various tissues and under exogenous salicylic acid (SA), methyl jasmonate (MeJA), and PWN infection. Six genes (PmNAC1, PmNAC8, PmNAC9, PmNAC17, PmNAC18, and PmNAC20) were significantly up-regulated by both hormonal treatment and PWN infection, implying their involvement in JA/SA-mediated immune pathways. Functional characterization showed PmNAC8 is a nuclear-localized transcription factor with autoactivation activity. Furthermore, transient overexpression of PmNAC8 in Nicotiana benthamiana induced reactive oxygen species (ROS) accumulation and necrotic lesions. Collectively, these results elucidate NAC-mediated defense responses to PWN infection in P. massoniana and identify candidate genes for developing PWD-resistant pine varieties.
Keywords: Bursaphelenchus xylophilus; NAC family; hormones; pine wilt disease; subcellular localization; transcription factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Tang F., Zhou Y., Feng J., Li J., Feng J., Lv Y., Mao Y., Wang Y., Deng P., Bai Y. Important Role of Pinus Massoniana Mixed Forests in Enhancing Soil Carbon Stocks in Degraded Forests in Southern China. CATENA. 2025;250:108792. doi: 10.1016/j.catena.2025.108792. - DOI
-
- Xu S., Huang W., Wang D., Zhang B., Sun H., Yan J., Ding J., Ma X. Risk Assessment of Carbon Stock Loss in Chinese Forests Due to Pine Wood Nematode Invasion. Forests. 2025;16:315. doi: 10.3390/f16020315. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

