Celery and Spinach Flavonoid-Rich Extracts Enhance Phytoalexin Production in Powdery Mildew-Infected Cucumber Leaves
- PMID: 40805762
- PMCID: PMC12349301
- DOI: 10.3390/plants14152414
Celery and Spinach Flavonoid-Rich Extracts Enhance Phytoalexin Production in Powdery Mildew-Infected Cucumber Leaves
Abstract
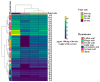
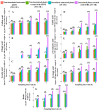
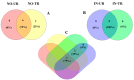
Phytoalexins are antimicrobial compounds of diverse chemical classes whose production is triggered in plants in response to pathogen infection. This study demonstrated that spraying with a celery flavonoid-rich extract (CFRE) or a spinach flavonoid-rich extract (SFRE) enhanced the production of phytoalexins in cucumber leaves artificially infected with powdery mildew incited by Podosphaera fusca. High-performance liquid chromatographic (HPLC) analysis revealed a noticeable increase in the content of phenolic acids, including caffeic acid, ellagic acid, ferulic acid, gallic acid, p-coumaric acid, and syringic acid, as well as the flavonoid rutin in both non-inoculated and inoculated leaves of cucumber seedlings treated with CFRE and SFRE, compared to healthy untreated leaves used as a control. Fluorescence microscopy revealed the accumulation of phenolic acid compounds in chloroplasts and at the periphery of epidermal cells. Overall, results suggest the reduced severity of P. fusca infection following the application of CFRE and SFRE in cucumber leaves could be due, at least in part, to the production of phytoalexins of polyphenolic nature. These findings provide insights into the mechanisms of systemic resistance induced by CFRE and SFRE. Moreover, they confirm these two natural flavonoid-rich products could be promising alternatives to synthetic chemical fungicides for the safe and ecofriendly control of cucumber powdery mildew.
Keywords: Apium graveolens; Cucumis sativus; Spinacia oleracea; flavonoid-rich plant extracts; phenolic compounds; phytoalexins; powdery mildew.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Fukino N., Yoshioka Y., Sugiyama M., Sakata Y., Matsumoto S. Identification and validation of powdery mildew (Podosphaera xanthii)-resistant loci in recombinant inbred lines of cucumber (Cucumis sativus L.) Mol. Breed. 2013;32:267–277. doi: 10.1007/s11032-013-9867-3. - DOI
-
- Romero-Contreras Y.J., Gonzalez-Serrano F., Formey D., Aragon W., Chacón F.I., Torres M., Cevallos M.A., Rafael Dib J., Rebollar E.A., Serrano M. Amphibian skin bacteria display antifungal activity and induce plant defense mechanisms against Botrytis cinerea. Front. Plant Sci. 2024;15:1392637. doi: 10.3389/fpls.2024.1392637. - DOI - PMC - PubMed
-
- Nie J., Yuan Q., Zhang W., Pan J. Genetics, resistance mechanism, and breeding of powdery mildew resistance in cucumbers (Cucumis sativus L.) Hortic. Plant J. 2023;9:603–615. doi: 10.1016/j.hpj.2023.05.013. - DOI
LinkOut - more resources
Full Text Sources

