A Novel Cysteine Protease from Phytolacca americana Cleaves Pokeweed Antiviral Protein Generating Bioactive Fragments
- PMID: 40805790
- PMCID: PMC12349160
- DOI: 10.3390/plants14152441
A Novel Cysteine Protease from Phytolacca americana Cleaves Pokeweed Antiviral Protein Generating Bioactive Fragments
Abstract
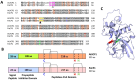
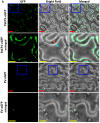
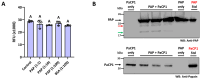
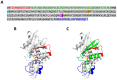
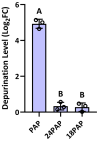
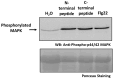
The apoplast is often the first point of contact between plant cells and invading pathogens, serving as an important site for defense signaling. Pokeweed antiviral protein (PAP), a ribosome-inactivating protein from Phytolacca americana (pokeweed), is localized to the apoplast and is hypothesized to accompany a pathogen to the cytosol, where it would inactivate host ribosomes to prevent pathogen spread. However, it is not known whether PAP interacts with other proteins in the apoplast. In this study, we identified Phytolacca americana cysteine protease 1 (PaCP1), an extracellular cysteine protease, as a novel PAP interactor. Sequence and structural analyses classified PaCP1 as a member of the C1A subfamily of papain-like cysteine proteases. Immunoprecipitation, mass spectrometry, and yeast two-hybrid analysis showed that PAP specifically binds the mature, active form of PaCP1. Curiously, PaCP1 cleaves PAP at its N- and C-termini, generating peptides that enhance MAPK phosphorylation in pokeweed leaves, indicating their potential role in stress signaling. PaCP1 processing of PAP to generate bioactive peptides diversifies the function of a ribosome-inactivating protein beyond its canonical inhibition of translation. Our findings present a novel extracellular role for PAP and advance our understanding of how protein interactions in the apoplast contribute to plant immune responses.
Keywords: RNA N-glycosylase; apoplast; cysteine protease; pokeweed antiviral protein; ribosome-inactivating protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
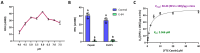


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Autistic Students' Experiences of Employment and Employability Support while Studying at a UK University.Autism Adulthood. 2025 Apr 3;7(2):212-222. doi: 10.1089/aut.2024.0112. eCollection 2025 Apr. Autism Adulthood. 2025. PMID: 40309023
-
Enteroviral 3C protease cleaves N4BP1 to impair the host inflammatory response.J Virol. 2025 Jan 31;99(1):e0175824. doi: 10.1128/jvi.01758-24. Epub 2024 Dec 10. J Virol. 2025. PMID: 39655957 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The quantity, quality and findings of network meta-analyses evaluating the effectiveness of GLP-1 RAs for weight loss: a scoping review.Health Technol Assess. 2025 Jun 25:1-73. doi: 10.3310/SKHT8119. Online ahead of print. Health Technol Assess. 2025. PMID: 40580049 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

