Abscisic Acid Metabolizing Rhodococcus sp. Counteracts Phytopathogenic Effects of Abscisic Acid Producing Botrytis sp. on Sunflower Seedlings
- PMID: 40805791
- PMCID: PMC12349427
- DOI: 10.3390/plants14152442
Abscisic Acid Metabolizing Rhodococcus sp. Counteracts Phytopathogenic Effects of Abscisic Acid Producing Botrytis sp. on Sunflower Seedlings
Abstract
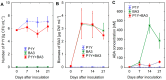
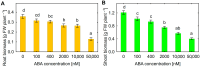
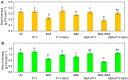
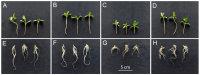
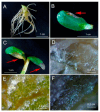
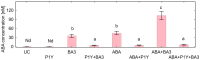
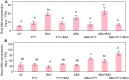
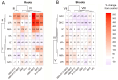
One of the important traits of many plant growth-promoting rhizobacteria (PGPR) is the biocontrol of phytopathogens. Some PGPR metabolize phytohormone abscisic acid (ABA); however, the role of this trait in plant-microbe interactions is scarcely understood. Phytopathogenic fungi produce ABA and use this property as a negative regulator of plant resistance. Therefore, interactions between ABA-producing necrotrophic phytopathogen Botrytis sp. BA3 with ABA-metabolizing rhizobacterium Rhodococcus sp. P1Y were studied in a batch culture and in gnotobiotic hydroponics with sunflower seedlings. Rhizobacterium P1Y possessed no antifungal activity against BA3 and metabolized ABA, which was synthesized by BA3 in vitro and in associations with sunflower plants infected with this fungus. Inoculation with BA3 and the application of exogenous ABA increased the root ABA concentration and inhibited root and shoot growth, suggesting the involvement of this phytohormone in the pathogenesis process. Strain P1Y eliminated negative effects of BA3 and exogenous ABA on root ABA concentration and plant growth. Both microorganisms significantly modulated the hormonal status of plants, affecting indole-3-acetic, salicylic, jasmonic and gibberellic acids, as well as cytokinins concentrations in sunflower roots and/or shoots. The hormonal effects were complex and could be due to the production of phytohormones by microorganisms, changes in ABA concentrations and multiple levels of crosstalk in hormone networks regulating plant defense. The results suggest the counteraction of rhizobacteria to ABA-producing phytopathogenic fungi through the metabolism of fungal ABA. This expands our understanding of the mechanisms related to the biocontrol of phytopathogens by PGPR.
Keywords: Botrytis; PGPR; abscisic acid; biocontrol; phytohormones; phytopathogens.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Synergistic Effect of Melatonin and Lysinibacillus fusiformis L. (PLT16) to Mitigate Drought Stress via Regulation of Hormonal, Antioxidants System, and Physio-Molecular Responses in Soybean Plants.Int J Mol Sci. 2023 May 9;24(10):8489. doi: 10.3390/ijms24108489. Int J Mol Sci. 2023. PMID: 37239837 Free PMC article.
-
Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth.Plant Physiol Biochem. 2014 Jan;74:84-91. doi: 10.1016/j.plaphy.2013.10.032. Epub 2013 Nov 1. Plant Physiol Biochem. 2014. PMID: 24270514
-
Phytohormones and related genes function as physiological and molecular switches regulating water stress response in the sunflower.Physiol Mol Biol Plants. 2024 Aug;30(8):1277-1295. doi: 10.1007/s12298-024-01497-8. Epub 2024 Aug 5. Physiol Mol Biol Plants. 2024. PMID: 39184555 Free PMC article.
-
From Hormones to Harvests: A Pathway to Strengthening Plant Resilience for Achieving Sustainable Development Goals.Plants (Basel). 2025 Jul 27;14(15):2322. doi: 10.3390/plants14152322. Plants (Basel). 2025. PMID: 40805671 Free PMC article. Review.
-
Unveiling the secrets of abiotic stress tolerance in plants through molecular and hormonal insights.3 Biotech. 2024 Oct;14(10):252. doi: 10.1007/s13205-024-04083-7. Epub 2024 Sep 26. 3 Biotech. 2024. PMID: 39345964 Review.
References
-
- El-Saadony M.T., Saad A.M., Soliman S.M., Salem H.M., Ahmed A.I., Mahmood M., El-Tahan A.M., Ebrahim A.A.M., Abd El-Mageed T.A., Negm S.H., et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022;13:923880. doi: 10.3389/fpls.2022.923880. - DOI - PMC - PubMed
-
- Meena M., Swapnil P., Divyanshu K., Kumar S., Harish, Tripathi Y.N., Zehra A., Marwal A., Upadhyay R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020;60:828–861. doi: 10.1002/jobm.202000370. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

