Pharmacoeconomic Profiles of Advanced Therapy Medicinal Products in Rare Diseases: A Systematic Review
- PMID: 40805927
- PMCID: PMC12345686
- DOI: 10.3390/healthcare13151894
Pharmacoeconomic Profiles of Advanced Therapy Medicinal Products in Rare Diseases: A Systematic Review
Abstract
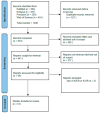
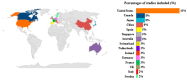
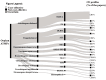
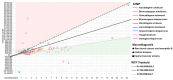
Background and aim: Advanced Therapy Medicinal Products (ATMPs) are innovative drugs based on genes, tissues, or cells that target rare and severe diseases. ATMPs have shown promising clinical outcomes but are associated with high costs, raising questions about cost-effectiveness. Hence, this systematic review aims to analyze the cost-effectiveness and cost-utility profiles of the European Medicines Agency-authorized ATMPs for treating rare diseases. Methods: A systematic review was conducted following PRISMA guidelines. Studies were identified by searching PubMed, Embase, Web of Science, and ProQuest scientific databases. Economic evaluations reporting incremental cost-effectiveness/utility ratios (ICERs/ICURs) for ATMPs were included. Costs were standardized to 2023 Euros, and a cost-effectiveness plane was constructed to evaluate the results against willingness-to-pay (WTP) thresholds of EUR 50,000, EUR 100,000, and EUR 150,000 per QALY, as part of a sensitivity analysis. Results: A total of 61 studies met the inclusion criteria. ATMPs for rare blood diseases, such as tisagenlecleucel and axicabtagene ciloleucel, were found to be cost-effective in a majority of studies, with incremental QALYs ranging from 1.5 to 10 per patient over lifetime horizon. Tisagenlecleucel demonstrated a positive cost-effectiveness profile in the treatment of acute lymphoblastic leukemia (58%), while axicabtagene ciloleucel showed a positive profile in the treatment of diffuse large B-cell lymphoma (85%). Onasemnogene abeparvovec for spinal muscular atrophy (SMA) showed uncertain cost-effectiveness results, and voretigene neparvovec for retinal diseases was not cost-effective in 40% of studies, with incremental QALYs around 1.3 and high costs exceeding the WTP threshold set. Conclusions: ATMPs in treating rare diseases show promising economic potential, but cost-effectiveness varies across indications. Policymakers must balance innovation with system sustainability, using refined models and the long-term impact on patient outcomes.
Keywords: ATMP; Advanced Therapy Medicinal Products; cost-effectiveness; economic evaluation; gene therapy; rare diseases.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Figures
Similar articles
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.Health Technol Assess. 2007 Nov;11(47):iii-iv, ix-248. doi: 10.3310/hta11470. Health Technol Assess. 2007. PMID: 17999842
-
Clinical effectiveness and cost-effectiveness of second- and third-generation left ventricular assist devices as either bridge to transplant or alternative to transplant for adults eligible for heart transplantation: systematic review and cost-effectiveness model.Health Technol Assess. 2013 Nov;17(53):1-499, v-vi. doi: 10.3310/hta17530. Health Technol Assess. 2013. PMID: 24280231 Free PMC article.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
References
-
- European Medicine Agency CAT Quarterly Highlights and Approved ATMPs. 2024. [(accessed on 28 October 2024)]. Available online: https://www.ema.europa.eu/en/documents/committee-report/cat-quarterly-hi....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

