A Refined Carbohydrate-Rich Diet Reduces Vascular Reactivity Through Endothelial Oxidative Stress and Increased Nitric Oxide: The Involvement of Inducible Nitric Oxide Synthase
- PMID: 40805981
- PMCID: PMC12348616
- DOI: 10.3390/nu17152395
A Refined Carbohydrate-Rich Diet Reduces Vascular Reactivity Through Endothelial Oxidative Stress and Increased Nitric Oxide: The Involvement of Inducible Nitric Oxide Synthase
Abstract
Background/objectives: The consumption of refined carbohydrates has increased globally. It is associated with inflammation and oxidative stress, both recognized as risk factors for cardiovascular disease. This study investigated the effects of a refined carbohydrate-rich diet on the vascular reactivity of rat aorta.
Methods: We acclimatized adult male Wistar rats for two weeks and then randomly assigned them to two experimental groups: a control (CT) group and a high-carbohydrate diet (HCD) group. The CT group received standard laboratory chow for 15 days, while the HCD group received a diet composed of 45% sweetened condensed milk, 10% refined sugar, and 45% standard chow. After the dietary exposure period, we evaluated the vascular reactivity of aortic rings, gene expression related to inflammation, superoxide dismutase activity, and biochemical parameters, including cholesterol, triglycerides, fasting glucose, and glucose and insulin tolerance.
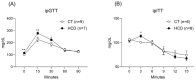
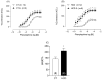
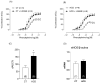
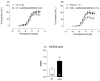
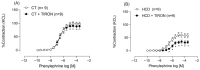
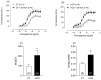
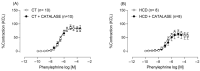
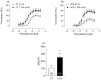
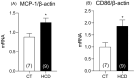
Results: The results demonstrate a reduction in vascular reactivity caused by endothelial alterations, including increased NO production, which was observed as higher vasoconstriction in the presence of L-NAME and aminoguanidine and upregulation of iNOS gene expression. In addition, increased production of free radicals, such as O2-, was observed, as well as immune markers like MCP-1 and CD86 in the HCD group. Additionally, the HCD group showed an increase in the TyG index, suggesting early metabolic impairment. GTT and ITT results revealed higher glycemic levels, indicating early signs of insulin resistance.
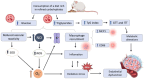
Conclusions: These findings indicate that short-term consumption of a refined carbohydrate-rich diet may trigger oxidative stress and endothelial dysfunction, thereby increasing the risk of cardiovascular complications.
Keywords: aorta; inflammation; oxidative stress; refined carbohydrate-rich diet; vascular reactivity.
Conflict of interest statement
The authors declare no conflicts of interest that could have influenced the work reported in this article.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Neurocognition in youth with versus without prediabetes.medRxiv [Preprint]. 2025 Jun 23:2025.06.23.25330130. doi: 10.1101/2025.06.23.25330130. medRxiv. 2025. PMID: 40666321 Free PMC article. Preprint.
-
A Plant-Based Dietary Supplement Exhibits Significant Effects on Markers of Oxidative Stress, Inflammation, and Immune Response in Subjects Recovering from Respiratory Viral Infection: A Randomized, Double-Blind Clinical Study Using Vitamin C as a Positive Control.Int J Mol Sci. 2025 May 29;26(11):5209. doi: 10.3390/ijms26115209. Int J Mol Sci. 2025. PMID: 40508019 Free PMC article. Clinical Trial.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- World Health Organization Cardiovascular Diseases (CVDs) [(accessed on 17 February 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

