A 3-Week Inpatient Rehabilitation Programme Improves Body Composition in People with Cystic Fibrosis with and Without Elexacaftor/Tezacaftor/Ivacaftor Therapy
- PMID: 40806025
- PMCID: PMC12348923
- DOI: 10.3390/nu17152439
A 3-Week Inpatient Rehabilitation Programme Improves Body Composition in People with Cystic Fibrosis with and Without Elexacaftor/Tezacaftor/Ivacaftor Therapy
Abstract
Background: The introduction of cystic fibrosis transmembrane conductance regulator modulators, especially the triple therapy elexacaftor, tezacaftor, ivacaftor (ETI), has improved outcomes in people with cystic fibrosis (pwCF), reducing underweight but increasing overweight rates.
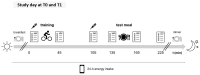
Objectives: This study investigates the effect of ETI on appetite control, body composition, and energy balance during a 3-week inpatient rehabilitation programme with regular exercise.
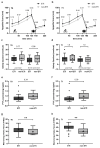
Methods: In 54 pwCF (38 on ETI, 16 without ETI), changes in body composition (fat mass index, FMI; fat-free mass index, FFMI) and energy balance (calculated from body composition changes) were assessed. Appetite control was evaluated via plasma peptide YY (PYY) levels and post-exercise meal energy intake.
Results: The programme significantly increased BMI (+0.3 ± 0.1 kg/m2; CI 0.1-0.4) and energy balance (+4317 ± 1976 kcal/3 weeks), primarily through FFMI gains (+0.3 ± 0.1 kg/m2; CI 0.1-0.4). Despite higher post-exercise meal energy intake and a tendency towards lower PYY levels in the ETI group, changes in body composition and energy balance did not differ between groups. This is explained by a higher prevalence of exocrine pancreatic insufficiency in the ETI group (92% vs. 50%, p < 0.001). Small sample sizes limit the interpretation of data on appetite control and energy intake.
Conclusions: A 3-week inpatient rehabilitation programme improved body composition in pwCF, without resulting in a more positive energy balance with ETI therapy. This is due to a higher prevalence of pancreatic insufficiency in this group.
Keywords: CF rehabilitation; Elexacaftor/Tezacaftor/Ivacaftor; appetite control; body composition; energy balance; triple CFTR modulators.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2023 Nov 20;11(11):CD010966. doi: 10.1002/14651858.CD010966.pub4. Cochrane Database Syst Rev. 2023. PMID: 37983082 Free PMC article.
-
Dietary intake remains unchanged while nutritional status improves in children and adults with cystic fibrosis on Elexacaftor/Tezacaftor/Ivacaftor.Clin Nutr. 2025 Jul;50:76-82. doi: 10.1016/j.clnu.2025.04.027. Epub 2025 Apr 30. Clin Nutr. 2025. PMID: 40378731
-
Evaluation of elexacaftor-tezacaftor-ivacaftor treatment in individuals with cystic fibrosis and CFTRN1303K in the USA: a prospective, multicentre, open-label, single-arm trial.Lancet Respir Med. 2024 Dec;12(12):947-957. doi: 10.1016/S2213-2600(24)00205-4. Epub 2024 Aug 26. Lancet Respir Med. 2024. PMID: 39208836
-
Population Pharmacokinetics of Elexacaftor, Tezacaftor and Ivacaftor in a Real-World Cohort of Adults with Cystic Fibrosis.Clin Pharmacokinet. 2025 Jun;64(6):959-971. doi: 10.1007/s40262-025-01516-1. Epub 2025 May 22. Clin Pharmacokinet. 2025. PMID: 40405059
-
Pharmacokinetics of Ivacaftor, Tezacaftor, Elexacaftor, and Lumacaftor in Special Cystic Fibrosis Populations: A Systematic Review.Clin Pharmacokinet. 2025 Jul;64(7):999-1046. doi: 10.1007/s40262-025-01507-2. Epub 2025 May 21. Clin Pharmacokinet. 2025. PMID: 40399734 Free PMC article.
References
-
- Naehrlich L., Burkhart M., Basler C., Dittrich A.-M., Ellemunter H., Hebestreit H., Nitsche O., Held I., Smaczny C., Sutharsan S. German Cystic Fibrosis Registry-Annual Report 2023. Mukoviszidose e.V. & Mukoviszidose Institut GmbH; Bonn, Germany: 2024.
-
- European Medicines Agency Kalydeco. [(accessed on 9 July 2024)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kalydeco#authorisation....
Grants and funding
LinkOut - more resources
Full Text Sources

