The Shuttling of Methyl Groups Between Folate and Choline Pathways
- PMID: 40806080
- PMCID: PMC12348172
- DOI: 10.3390/nu17152495
The Shuttling of Methyl Groups Between Folate and Choline Pathways
Abstract
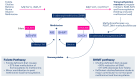
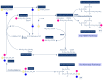
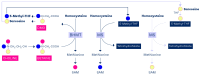
Methyl groups can be obtained either from the diet (labile methyl groups) or produced endogenously (methylneogenesis) via one-carbon (C1-) metabolism as S-adenosylmethionine (SAM). The essential nutrients folate and choline (through betaine) are metabolically entwined to feed their methyl groups into C1-metabolism. A choline-deficient diet in rats produces a 31-40% reduction in liver folate content, 50% lower hepatic SAM levels, and a doubling of plasma homocysteine. Similarly, folate deficiency results in decreased total hepatic choline. Thus, sufficient intakes of both folate and choline (or betaine) contribute to safeguarding the methyl balance in the body. A significant amount of choline (as phosphatidylcholine) is produced in the liver via the SAM-dependent phosphatidylethanolamine methyltransferase. Experimental studies using diets deficient in several methyl donors have shown that supplemental betaine was able to rescue not only plasma betaine but also plasma folate. Fasting plasma homocysteine concentrations are mainly determined by folate intake or status, while the effect of choline or betaine on fasting plasma homocysteine is minor. This appears to contradict the finding that approximately 50% of cellular SAM is provided via the betaine-homocysteine methyltransferase (BHMT) pathway, which uses dietary choline (after oxidation to betaine) or betaine to convert homocysteine to methionine and then to SAM. However, it has been shown that the relative contribution of choline and betaine to cellular methylation is better reflected by measuring plasma homocysteine after a methionine load test. Choline or betaine supplementation significantly lowers post-methionine load homocysteine, whereas folate supplementation has a minor effect on post-methionine load homocysteine concentrations. This review highlights the interactions between folate and choline and the essentiality of choline as a key player in C1-metabolism. We further address some areas of interest for future work.
Keywords: S-adenosylmethionine; betaine; choline; folate; homocysteine; metabolism; methionine-load test; methyl donor.
Conflict of interest statement
J.B. is employed by Balchem Corporation. R.O. received honoraria for lectures and served as an advisory board member for P&G Health GmbH, Wörwag Pharma GmbH, Balchem Corporation, Merck & Cie, and HIPP GmbH.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
One-carbon metabolite supplementation increases vitamin B12, folate, and methionine cycle metabolites in beef heifers and fetuses in an energy dependent manner at day 63 of gestation.J Anim Sci. 2024 Jan 3;102:skae202. doi: 10.1093/jas/skae202. J Anim Sci. 2024. PMID: 39028746 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- da Mota J.C.N., Ribeiro A.A., Carvalho L.M., Esteves G.P., Sieczkowska S.M., Goessler K.F., Gualano B., Nicoletti C.F. Impact of Methyl-Donor Micronutrient Supplementation on DNA Methylation Patterns: A Systematic Review and Meta-Analysis of in vitro, Animal, and Human Studies. Lifestyle Genom. 2023;16:192–213. doi: 10.1159/000533193. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

