Impact of Omega-3 and Vitamin D Supplementation on Bone Turnover Markers in Children with Leukemia: Follow-Up During and After Supplementation
- PMID: 40806109
- PMCID: PMC12348430
- DOI: 10.3390/nu17152526
Impact of Omega-3 and Vitamin D Supplementation on Bone Turnover Markers in Children with Leukemia: Follow-Up During and After Supplementation
Abstract
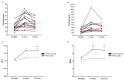
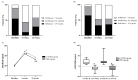
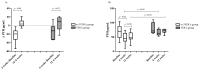
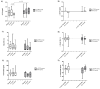
Background/Objective: In patients with acute lymphoblastic leukemia (ALL), it has been demonstrated that the treatment has a negative effect on bone health. The n-3 polyunsaturated fatty acids (LCPUFAs-ω3) may attenuate bone resorption. We evaluated the effects of LCPUFAs-ω3, vitamin D, and calcium supplementation on bone turnover markers and changes in vitamin D concentrations during 6 weeks of supplementation and during 6 weeks of post-intervention follow-up in pediatric patients with ALL. Methods: Thirty-six pediatric patients with ALL were randomly assigned to the ω-3VDCa group (100 mg/kg/d LCPUFAs-ω3 + 4000 IU vitamin D + 1000 mg calcium) or the VDCa group (4000 IU vitamin D + 1000 mg calcium) for 6 weeks. Blood samples were collected to determine 25(OH)D, PTH, ICTP, and TRAP-5b (biomarkers of bone resorption) and osteocalcin (OC, a biomarker of bone production) levels at baseline, 6 weeks, and 12 weeks after supplementation. The 25(OH)D analysis was performed using ultra-high-performance liquid chromatography coupled to a mass spectrometer, and PTH and bone turnover markers were measured by ELISA. Results: The 25(OH)D concentration increased in both groups (ω3VDCa group: 19.4 ng/mL vs. 44.0 ng/mL, p < 0.0001; VDCa group: 15.3 ng/mL vs. 42.8 ng/mL, p = 0.018) and remained significantly higher at 12 weeks. At 12 weeks, ICTP showed lower concentrations in the ω-3VDCa group than in the VDCa group (0.74 ng/mL vs. 1.05 ng/mL, p = 0.024). Conclusions: Combined omega-3 and 4000 IU vitamin D supplementation for 6 weeks had a positive effect on bone health, as indicated by serum ICTP, with no effect on serum 25(OH)D levels over vitamin D supplementation alone.
Keywords: acute lymphoblastic leukemia; bone turnover markers; children; dietary supplements; n-3 polyunsaturated fatty acids; vitamin D.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article to disclose.
Figures

References
-
- Velentza L., Zaman F., Sävendahl L. Bone health in glucocorticoid-treated childhood acute lymphoblastic leukemia. Crit. Rev. Oncol. Hematol. 2021;168:103492. - PubMed
-
- Atilano-Miguel S., Barbosa-Cortés L., Ortiz-Muñiz R., Maldonado-Hernández J., Martin-Trejo J.A., Rodríguez-Cruz M., Balcázar-Hernández L., Solís-Labastida K.A., Bautista-Martínez B.A., Juárez-Moya A., et al. Changes in RANKL, OPG, and 25(OH)D Levels in Children with Leukemia from Diagnosis to Remission. Cancers. 2024;16:2811. doi: 10.3390/cancers16162811. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

