Eurycomanone Blocks TGF-β1-Induced Epithelial-to-Mesenchymal Transition, Migration, and Invasion Pathways in Human Non-Small Cell Lung Cancer Cells by Targeting Smad and Non-Smad Signaling
- PMID: 40806251
- PMCID: PMC12345748
- DOI: 10.3390/ijms26157120
Eurycomanone Blocks TGF-β1-Induced Epithelial-to-Mesenchymal Transition, Migration, and Invasion Pathways in Human Non-Small Cell Lung Cancer Cells by Targeting Smad and Non-Smad Signaling
Abstract
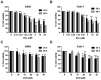
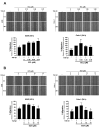
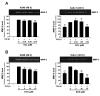
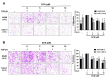
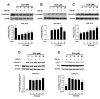
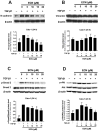
Non-small cell lung cancer (NSCLC) is a predominant form of lung cancer that is often diagnosed at an advanced metastatic stage. The processes of cancer cell migration and invasion involve epithelial-to-mesenchymal transition (EMT), which is crucial for metastasis. Targeting cancer aggressiveness with effective plant compounds has gained attention as a potential adjuvant therapy. Eurycomanone (ECN), a bioactive quassinoid found in the root of Eurycoma longifolia Jack, has demonstrated anti-cancer activity against various carcinoma cell lines, including human NSCLC cells. This study aimed to investigate the in vitro effects of ECN on the migration and invasion of human NSCLC cells and to elucidate the mechanisms by which ECN modulates the EMT in these cells. Non-toxic doses (≤IC20) of ECN were determined using the MTT assay on two human NSCLC cell lines: A549 and Calu-1. The results from wound healing and transwell migration assays indicated that ECN significantly suppressed the migration of both TGF-β1-induced A549 and Calu-1 cells. ECN exhibited a strong anti-invasive effect, as its non-toxic doses significantly suppressed the TGF-β1-induced invasion of NSCLC cells through Matrigel and decreased the secretion of MMP-2 from these cancer cells. Furthermore, ECN could affect the TGF-β1-induced EMT process in various ways in NSCLC cells. In TGF-β1-induced A549 cells, ECN significantly restored the expression of E-cadherin by inhibiting the Akt signaling pathway. Conversely, in Calu-1, ECN reduced the aggressive phenotype by decreasing the expression of the mesenchymal protein N-cadherin and inhibiting the TGF-β1/Smad pathway. In conclusion, this study demonstrated the anti-invasive activity of eurycomanone from E. longifolia Jack in human NSCLC cells and provided insights into its mechanism of action by suppressing the effects of TGF-β1 signaling on the EMT program. These findings offer scientific evidence to support the potential of ECN as an alternative therapy for metastatic NSCLC.
Keywords: epithelial-to-mesenchymal transition; eurycomalactone; eurycomanone; invasion; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
YiQiChuTan formula (YQCTF) inhibit the progression of non-small cell lung cancer via down-regulating EGFR/ITGB2 signaling: Triangulated evidence from network pharmacology, proteomic profiling, and experimental validation.Phytomedicine. 2025 Aug;144:156950. doi: 10.1016/j.phymed.2025.156950. Epub 2025 Jun 4. Phytomedicine. 2025. PMID: 40505482
-
TGF-β1 exposure induces epithelial to mesenchymal transition both in CSCs and non-CSCs of the A549 cell line, leading to an increase of migration ability in the CD133+ A549 cell fraction.Cell Death Dis. 2013 May 2;4(5):e620. doi: 10.1038/cddis.2013.144. Cell Death Dis. 2013. PMID: 23640462 Free PMC article.
-
The Role of Twisted Gastrulation 1 (TWSG1) Gene in TGF-β Signaling Linked to Cancer: A Comprehensive Review.Asian Pac J Cancer Prev. 2025 Apr 1;26(4):1129-1138. doi: 10.31557/APJCP.2025.26.4.1129. Asian Pac J Cancer Prev. 2025. PMID: 40302064 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Guo H., Li H., Zhu L., Feng J., Huang X., Baak J.P.A. “How long have I got?” in stage IV NSCLC patients with at least 3 months up to 10 years survival, accuracy of long-, intermediate-, and short-term survival prediction is not good enough to answer this question. Front. Oncol. 2021;11:761042. doi: 10.3389/fonc.2021.761042. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

