AI-Powered Mice Behavior Tracking and Its Application for Neuronal Manifold Analysis Based on Hippocampal Ensemble Activity in an Alzheimer's Disease Mice Model
- PMID: 40806314
- PMCID: PMC12346443
- DOI: 10.3390/ijms26157180
AI-Powered Mice Behavior Tracking and Its Application for Neuronal Manifold Analysis Based on Hippocampal Ensemble Activity in an Alzheimer's Disease Mice Model
Abstract
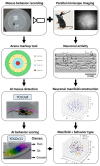
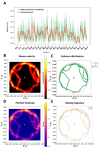
Investigating brain area functions requires advanced technologies, but meaningful insights depend on correlating neural signals with behavior. Traditional mice behavior annotation methods, including manual and semi-automated approaches, are limited by subjectivity and time constraints. To overcome these limitations, our study employs the YOLO neural network for precise mice tracking and composite RGB frames for behavioral scoring. Our model, trained on over 10,000 frames, accurately classifies sitting, running, and grooming behaviors. Additionally, we provide statistical metrics and data visualization tools. We further combined AI-powered behavior labeling to examine hippocampal neuronal activity using fluorescence microscopy. To analyze neuronal circuit dynamics, we utilized a manifold analysis approach, revealing distinct functional patterns corresponding to transgenic 5xFAD Alzheimer's model mice. This open-source software enhances the accuracy and efficiency of behavioral and neural data interpretation, advancing neuroscience research.
Keywords: Alzheimer’s disease; YOLO; artificial intelligence; behavioral classification; calcium imaging; locomotion; manifold; mice tracking; neuronal activity; neuronal network.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
DeePosit, an AI-based tool for detecting mouse urine and fecal depositions from thermal video clips of behavioral experiments.Elife. 2025 Aug 28;13:RP100739. doi: 10.7554/eLife.100739. Elife. 2025. PMID: 40874843 Free PMC article.
-
Degenerate mapping of environmental location presages deficits in object-location encoding and memory in the 5xFAD mouse model for Alzheimer's disease.Neurobiol Dis. 2023 Jan;176:105939. doi: 10.1016/j.nbd.2022.105939. Epub 2022 Dec 1. Neurobiol Dis. 2023. PMID: 36462718 Free PMC article.
-
Development of a Marmoset Apparatus for Automated Pulling to study cooperative behaviors.Elife. 2024 Oct 28;13:RP97088. doi: 10.7554/eLife.97088. Elife. 2024. PMID: 39466838 Free PMC article.
-
Nimodipine for primary degenerative, mixed and vascular dementia.Cochrane Database Syst Rev. 2001;(1):CD000147. doi: 10.1002/14651858.CD000147. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2002;(3):CD000147. doi: 10.1002/14651858.CD000147. PMID: 11279679 Updated.
-
Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2014 Jun 10;2014(6):CD008782. doi: 10.1002/14651858.CD008782.pub4. Cochrane Database Syst Rev. 2014. PMID: 24913723 Free PMC article.
References
-
- Im C., Seo J.M. A Review of Electrodes for the Electrical Brain Signal Recording. Biomed. Eng. Lett. 2016;6:104–112. doi: 10.1007/s13534-016-0235-1. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

