Extracellular Vesicles-Induced Cell Homing and Odontogenesis via microRNA Signaling for Dentin Regeneration
- PMID: 40806315
- PMCID: PMC12346742
- DOI: 10.3390/ijms26157182
Extracellular Vesicles-Induced Cell Homing and Odontogenesis via microRNA Signaling for Dentin Regeneration
Abstract
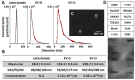
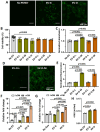
Reparative tertiary dentinogenesis requires the recruitment and odontogenic differentiation of dental pulp stem cells (DPSCs). Extracellular vesicles (EVs) as bioactive molecules have gained attention in regenerative medicine for their ability to mediate tissue repair through intercellular communication, influencing cell recruitment, proliferation, and differentiation. This study aimed to evaluate the effects of EVs on DPSC homing and odontogenic differentiation for dentin regeneration. DPSC-derived EVs were cultured in either growth (EV-G) or odontogenic differentiation (EV-O) conditions and isolated using a modified precipitation method. EVs were characterized by nanoparticle tracking analysis, scanning electron microscopy, antibody array, and cellular uptake assay. Treatment with 5 × 108 EVs/mL significantly enhanced DPSC chemotaxis and proliferation compared with a no-treatment control and a lower dosage of EV (5 × 107 EVs/mL). Gene expression and biochemical analyses revealed that EV-O up-regulated odontogenic markers including collagen type 1A1 (COL1A1), runt-related transcription factor 2 (RUNX2), and alkaline phosphatase (ALP). EV-O enhanced dentin regeneration by approximately 55% over vehicle controls in a rabbit partial dentinotomy/pulpotomy model. We identified key microRNAs (miR-21-5p, miR-221-3p, and miR-708-3p) in EV-O involved in cell homing and odontogenesis. In conclusion, our EV-based cell homing and odontogenic differentiation strategy has significant therapeutic potential for dentin regeneration.
Keywords: cell homing; dental pulp stem cells; dentin regeneration; dentinogenesis; extracellular vesicles; microRNA; odontogenesis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
The Hippo-YAP/β-catenin signaling axis coordinates odontogenic differentiation in dental pulp stem cells: Implications for dentin-pulp regeneration.PLoS One. 2025 Jun 26;20(6):e0326978. doi: 10.1371/journal.pone.0326978. eCollection 2025. PLoS One. 2025. PMID: 40570025 Free PMC article.
-
CRISPR-edited DPSCs constitutively expressing BDNF enhance dentin regeneration in injured teeth.Elife. 2025 Jul 9;14:RP105153. doi: 10.7554/eLife.105153. Elife. 2025. PMID: 40631557 Free PMC article.
-
Functional extracellular vesicles from SHEDs combined with gelatin methacryloyl promote the odontogenic differentiation of DPSCs for pulp regeneration.J Nanobiotechnology. 2024 May 17;22(1):265. doi: 10.1186/s12951-024-02542-0. J Nanobiotechnology. 2024. PMID: 38760763 Free PMC article.
-
Extracellular vesicles derived from Schwann cells to enhance bone and dental tissue regeneration: a literature review.J Nanobiotechnology. 2025 Jul 11;23(1):502. doi: 10.1186/s12951-025-03585-7. J Nanobiotechnology. 2025. PMID: 40646600 Free PMC article. Review.
-
Cellular dynamics and signalling mechanisms in dentine repair: A narrative review.Int Endod J. 2025 Sep;58(9):1354-1383. doi: 10.1111/iej.14261. Epub 2025 Jun 9. Int Endod J. 2025. PMID: 40491185 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

