Site-Specific Gut Microbiome Changes After Roux-en-Y Gastric Bypass in Rats: Effects of a Multicomponent Bovine Colostrum-Based Complex
- PMID: 40806321
- PMCID: PMC12347457
- DOI: 10.3390/ijms26157186
Site-Specific Gut Microbiome Changes After Roux-en-Y Gastric Bypass in Rats: Effects of a Multicomponent Bovine Colostrum-Based Complex
Abstract
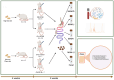
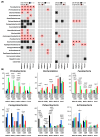
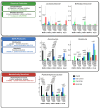
Roux-en-Y gastric bypass (RYGB) surgery induces profound gut microbiota alterations that may impact metabolic outcomes. This study investigated site-specific effects of a multicomponent bovine colostrum-honey-serviceberry (CHJ) complex on post-RYGB microbiome changes in obese rats. Twenty-nine Wistar rats underwent RYGB surgery with CHJ supplementation, followed by mucosal-associated microbiota analysis from five gastrointestinal segments using 16S rRNA sequencing and serum metabolite profiling. RYGB caused regional-specific changes: decreased alpha diversity, systematic Proteobacteria increases (31.2 ± 5.1% in duodenum), and reductions in SCFA-producing bacteria (Romboutsia, Roseburia). CHJ supplementation exhibited dual effects on the microbiome: restoration of beneficial bacteria (Lactobacillus, Bifidobacterium) in distal segments while concurrently promoting Enterobacteriaceae growth in proximal regions. CHJ also maintained alpha diversity levels of the mucosa-associated microbiota comparable to those observed in the control group. Disconnects emerged between predicted microbial functions and systemic metabolites: thiamine pathway activation accompanied 78.5% serum vitamin B1 reduction, indicating severe absorption deficits. Three distinct patterns emerged: pro-inflammatory (proximal), decolonization (widespread Helicobacteraceae loss), and restorative (selective CHJ-mediated recovery). Results demonstrate that post-RYGB dysbiosis exhibits profound regional heterogeneity requiring segment-specific interventions and highlight complex interactions between nutritional supplementation and surgically altered gut ecology in determining metabolic outcomes.
Keywords: Enterobacteriaceae; Roux-en-Y gastric bypass; SCFA-producing bacteria; bariatric surgery; bovine colostrum; dysbiosis; gut microbiome; metabolic syndrome; site specificity.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Impact of bariatric surgery on gut microbiota in obese patients: A systematic review.Indian J Gastroenterol. 2025 Aug;44(4):457-477. doi: 10.1007/s12664-025-01763-x. Epub 2025 Apr 12. Indian J Gastroenterol. 2025. PMID: 40220249
-
A microbial signature following bariatric surgery is robustly consistent across multiple cohorts.Gut Microbes. 2021 Jan-Dec;13(1):1930872. doi: 10.1080/19490976.2021.1930872. Gut Microbes. 2021. PMID: 34159880 Free PMC article.
-
The impact of early-life exposures on growth and adult gut microbiome composition is dependent on genetic strain and parent- of- origin.Microbiome. 2025 Jun 16;13(1):143. doi: 10.1186/s40168-025-02130-w. Microbiome. 2025. PMID: 40524209 Free PMC article.
-
Gut microbiota alterations and their role in the pathophysiology of obesity following bariatric surgery.Expert Rev Endocrinol Metab. 2025 Jul;20(4):291-305. doi: 10.1080/17446651.2025.2512551. Epub 2025 Jun 3. Expert Rev Endocrinol Metab. 2025. PMID: 40460250 Review.
-
Prepregnancy Roux-en-Y gastric bypass vs sleeve gastrectomy: a systematic review, pairwise, and network meta-analysis of obstetrical and neonatal outcomes.Am J Obstet Gynecol MFM. 2023 Jun;5(6):100914. doi: 10.1016/j.ajogmf.2023.100914. Epub 2023 Mar 7. Am J Obstet Gynecol MFM. 2023. PMID: 36889438
References
-
- Noubiap J.J., Nansseu J.R., Lontchi-Yimagou E., Nkeck J.R., Nyaga U.F., Ngouo A.T., Tounouga D.N., Tianyi F.-L., Foka A.J., Ndoadoumgue A.L., et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022;188:109924. doi: 10.1016/j.diabres.2022.109924. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

