Recent Advances in Engineering the Unfolded Protein Response in Recombinant Chinese Hamster Ovary Cell Lines
- PMID: 40806322
- PMCID: PMC12346540
- DOI: 10.3390/ijms26157189
Recent Advances in Engineering the Unfolded Protein Response in Recombinant Chinese Hamster Ovary Cell Lines
Abstract
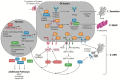
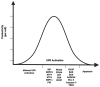
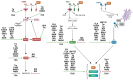
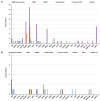
Chinese hamster ovary (CHO) cells are the most common protein production platform for glycosylated biopharmaceuticals due to their relatively efficient secretion systems, post-translational modification (PTM) machinery, and quality control mechanisms. However, high productivity and titer demands can overburden these processes. In particular, the endoplasmic reticulum (ER) can become overwhelmed with misfolded proteins, triggering the unfolded protein response (UPR) as evidence of ER stress. The UPR increases the expression of multiple genes/proteins, which are beneficial to protein folding and secretion. However, if the stressed ER cannot return to a state of homeostasis, a prolonged UPR results in apoptosis. Because ER stress poses a substantial bottleneck for secreting protein therapeutics, CHO cells are both selected for and engineered to improve high-quality protein production through optimized UPR and ER stress management. This is vital for optimizing industrial CHO cell fermentation. This review begins with an overview of common ER-stress related markers. Next, the optimal UPR profile of high-producing CHO cells is discussed followed by the context-dependency of a UPR profile for any given recombinant CHO cell line. Recent efforts to control and engineer ER stress-related responses in CHO cell lines through the use of various bioprocess operations and activation/inhibition strategies are elucidated. Finally, this review concludes with a discussion on future directions for engineering the CHO cell UPR.
Keywords: Chinese hamster ovary (CHO) cells; ER stress; biopharmaceuticals; synthetic biology; therapeutic proteins; unfolded protein response (UPR).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Monoclonal Antibodies Market Size Set to Hit USD 679.03 Bn by 2033. [(accessed on 17 May 2025)]. Available online: https://www.visionresearchreports.com/monoclonal-antibodies-market/38040.
-
- FDALabel. [(accessed on 17 May 2025)]; Available online: https://nctr-crs.fda.gov/fdalabel/ui/search.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

