Gene Monitoring in Obesity-Induced Metabolic Dysfunction in Rats: Preclinical Data on Breast Neoplasia Initiation
- PMID: 40806434
- PMCID: PMC12346978
- DOI: 10.3390/ijms26157296
Gene Monitoring in Obesity-Induced Metabolic Dysfunction in Rats: Preclinical Data on Breast Neoplasia Initiation
Abstract
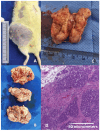
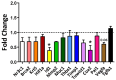
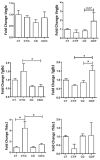
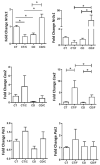
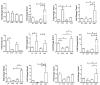
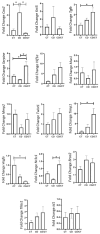
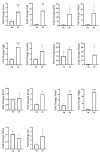
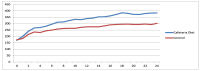
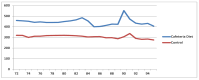
Obesity and metabolic dysfunction are established risk factors for luminal breast cancer, yet current preclinical models inadequately recapitulate the complex metabolic and immune interactions driving tumorigenesis. To develop and characterize an immunocompetent rat model of luminal breast cancer induced by chronic exposure to a cafeteria diet mimicking Western obesogenic nutrition, female rats were fed a cafeteria diet or standard chow from weaning. Metabolic parameters, plasma biomarkers (including leptin, insulin, IGF-1, adiponectin, and estrone), mammary gland histology, tumor incidence, and gene expression profiles were longitudinally evaluated. Gene expression was assessed by PCR arrays and qPCR. A subgroup underwent dietary reversal to assess the reversibility of molecular alterations. Cafeteria diet induced significant obesity (mean weight 426.76 g vs. 263.09 g controls, p < 0.001) and increased leptin levels without altering insulin, IGF-1, or inflammatory markers. Histological analysis showed increased ductal ectasia and benign lesions, with earlier fibroadenoma and luminal carcinoma development in diet-fed rats. Tumors exhibited luminal phenotype, low Ki67, and elevated PAI-1 expression. Gene expression alterations were time point specific and revealed early downregulation of ID1 and COX2, followed by upregulation of MMP2, THBS1, TWIST1, and PAI-1. Short-term dietary reversal normalized several gene expression changes. Overall tumor incidence was modest (~12%), reflecting early tumor-promoting microenvironmental changes rather than aggressive carcinogenesis. This immunocompetent cafeteria diet rat model recapitulates key metabolic, histological, and molecular features of obesity-associated luminal breast cancer and offers a valuable platform for studying early tumorigenic mechanisms and prevention strategies without carcinogen-induced confounders.
Keywords: breast cancer preclinical model; gene expression; immunocompetent rat model; luminal breast cancer; metabolic dysfunction; obesity; tumor microenvironment.
Conflict of interest statement
The authors declare no potential conflicts of interest. The authors confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal.
Figures




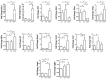
References
-
- Kolonin M.G. Role of adipose cells in tumor microenvironment. In: Benayahu D., Gefen A., editors. The Mechanobiology of Obesity and Related Diseases. Springer; Berlin/Heidelberg, Germany: 2014. pp. 271–294.
-
- Santander A.M., Lopez-Ocejo O., Casas O., Agostini T., Sanchez L., Lamas-Basulto E., Carrio R., Cleary M.P., Gonzalez-Perez R.R., Torroella-Kouri M. Paracrine interactions between adipocytes and tumor cells recruit and modify macrophages to the mammary tumor microenvironment: The role of obesity and inflammation in breast adipose tissue. Cancers. 2015;7:143–178. doi: 10.3390/cancers7010143. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

