Roles of Ion Channels in Oligodendrocyte Precursor Cells: From Physiology to Pathology
- PMID: 40806469
- PMCID: PMC12348010
- DOI: 10.3390/ijms26157336
Roles of Ion Channels in Oligodendrocyte Precursor Cells: From Physiology to Pathology
Abstract
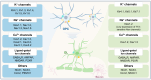
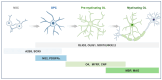
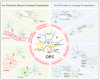
Oligodendrocyte precursor cells (OPCs) are a distinct and dynamic glial population that retain proliferative and migratory capacities throughout life. While traditionally recognized for differentiating into oligodendrocytes (OLs) and generating myelin to support rapid nerve conduction, OPCs are now increasingly appreciated for their diverse and non-canonical roles in the central nervous system (CNS), including direct interactions with neurons. A notable feature of OPCs is their expression of diverse ion channels that orchestrate essential cellular functions, including proliferation, migration, and differentiation. Given their widespread distribution across the CNS, OPCs are increasingly recognized as active contributors to the development and progression of various neurological disorders. This review aims to present a detailed summary of the physiological and pathological functions of ion channels in OPCs, emphasizing their contribution to CNS dysfunction. We further highlight recent advances suggesting that ion channels in OPCs may serve as promising therapeutic targets across a broad range of disorders, including, but not limited to, multiple sclerosis (MS), spinal cord injury, amyotrophic lateral sclerosis (ALS), psychiatric disorders, Alzheimer's disease (AD), and neuropathic pain (NP). Finally, we discuss emerging therapeutic strategies targeting OPC ion channel function, offering insights into potential future directions in the treatment of CNS diseases.
Keywords: central nervous system; ion channel; neurological diseases; oligodendrocyte precursor cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
C1ql1 expression in oligodendrocyte progenitor cells promotes oligodendrocyte differentiation.FEBS J. 2025 Jan;292(1):52-74. doi: 10.1111/febs.17256. Epub 2024 Sep 11. FEBS J. 2025. PMID: 39257292
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
-
Impact of Sex Differences in Oligodendrocytes and Their Progenitor Cells on the Pathophysiology of Neuropsychiatric Disorders.J Nippon Med Sch. 2025;92(3):226-233. doi: 10.1272/jnms.JNMS.2025_92-306. J Nippon Med Sch. 2025. PMID: 40603012 Review.
-
Oligodendrocyte progenitor cells' fate after neonatal asphyxia-Puzzling implications for the development of hypoxic-ischemic encephalopathy.Brain Pathol. 2024 Nov;34(6):e13255. doi: 10.1111/bpa.13255. Epub 2024 Mar 19. Brain Pathol. 2024. PMID: 38504469 Free PMC article.
-
Characterisation of GPR17-expressing oligodendrocyte precursors in human ischaemic lesions and correlation with reactive glial responses.J Pathol. 2025 Feb;265(2):226-243. doi: 10.1002/path.6381. Epub 2024 Dec 20. J Pathol. 2025. PMID: 39703181 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

