Nuclear Receptors in Bladder Cancer: Insights into miRNA-Mediated Regulation and Potential Therapeutic Implications
- PMID: 40806474
- PMCID: PMC12347959
- DOI: 10.3390/ijms26157340
Nuclear Receptors in Bladder Cancer: Insights into miRNA-Mediated Regulation and Potential Therapeutic Implications
Abstract
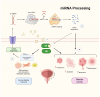
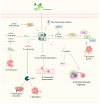
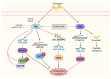
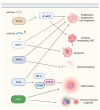
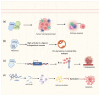
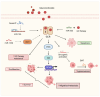
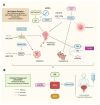
Nuclear receptors (NRs) are ligand-activated transcription factors that regulate gene expression and are involved in diverse physiological and pathological processes, including carcinogenesis. In bladder cancer (BCa), dysregulation of NR signaling pathways has been linked to tumor initiation, progression, therapy resistance, and immune evasion. Recent evidence highlights the intricate crosstalk between NRs and microRNAs (miRNAs), which are small non-coding RNAs that posttranscriptionally modulate gene expression. This review provides an integrated overview of the molecular interactions between key NRs and miRNAs in BCa. We investigated how miRNAs regulate NR expression and function and, conversely, how NRs influence miRNA biogenesis, thereby forming regulatory feedback loops that shape tumor behavior. Specific miRNA-NR interactions affecting epithelial-to-mesenchymal transition, metabolic reprogramming, angiogenesis, and chemoresistance are discussed in detail. Additionally, we highlight therapeutic strategies targeting NR-miRNA networks, including selective NR modulators, miRNA mimics and inhibitors, as well as RNA-based combinatorial approaches focusing on their utility as diagnostic biomarkers and personalized treatment targets. Understanding the molecular complexity of NR-miRNA regulation in BCa may open new avenues for improving therapeutic outcomes and advancing precision oncology in urological cancers.
Keywords: RNA-based therapy; bladder cancer; gene regulation; miRNAs; nuclear receptors; therapeutic response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection.J Virol. 2017 Mar 29;91(8):e02388-16. doi: 10.1128/JVI.02388-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148804 Free PMC article.
-
Harnessing the therapeutic potential of MicroRNAs and phytochemicals in modulating miRNA regulation during carcinogenesis.Biochem Biophys Res Commun. 2025 Sep 8;778:152381. doi: 10.1016/j.bbrc.2025.152381. Epub 2025 Jul 17. Biochem Biophys Res Commun. 2025. PMID: 40716307 Review.
-
MicroRNA and Alzheimer's disease: Diagnostic biomarkers and potential therapeutic targets.Neural Regen Res. 2025 Jul 5. doi: 10.4103/NRR.NRR-D-25-00002. Online ahead of print. Neural Regen Res. 2025. PMID: 40618273
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
MicroRNAs in Cancer Immunology: Master Regulators of the Tumor Microenvironment and Immune Evasion, with Therapeutic Potential.Cancers (Basel). 2025 Jun 27;17(13):2172. doi: 10.3390/cancers17132172. Cancers (Basel). 2025. PMID: 40647470 Free PMC article. Review.
References
-
- Kohada Y., Hayashi T., Hsi R.S., Yukihiro K., Sentani K., Goto K., Inoue S., Ohara S., Teishima J., Kajiwara M., et al. Recurrence- and progression-free survival in intermediate-risk non-muscle-invasive bladder cancer: The impact of conditional evaluation and subclassification. BJU Int. 2021;127:473–485. doi: 10.1111/bju.15209. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

