The Lysine at Position 177 Is Essential to Limit the Inhibitory Capacities of Sprouty4 Protein in Normal and Cancer-Derived Cells
- PMID: 40806483
- PMCID: PMC12347467
- DOI: 10.3390/ijms26157353
The Lysine at Position 177 Is Essential to Limit the Inhibitory Capacities of Sprouty4 Protein in Normal and Cancer-Derived Cells
Abstract
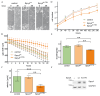
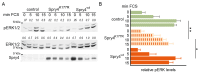
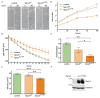
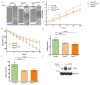
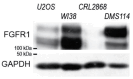
The Sprouty (Spry) proteins modulate signalling and regulate processes like cellular migration and proliferation. Here, we investigated a Spry4 alteration substituting a lysine at position 177 to an arginine, based on a mutation found in Kallmann syndrome, a genetically heterogeneous disease connected to reduced fibroblast growth factor receptor1 (FGFR) signalling. Using growth curves to evaluate proliferative and scratch assays to determine migrative capacities of the cells, in normal fibroblasts as well as in osteosarcoma-derived cells, we demonstrate that the modified Spry4K177R version hinders both processes, which the unaltered protein cannot do under the same conditions. The inhibition of these processes was accompanied by lower relative phospho-extracellular-signal-regulated kinases (pERK) levels in response to serum induction, indicating that activation of MAPK was less efficient. In contrast to the situation in these cells of mesenchymal origin, in lung cancer-derived cell lines both variants of Spry4 were able to interfere with proliferation of tested cells, and in the cells with elevated FGFR1 expression the Spry4 proteins with an alteration at codon 177 were even more effective. In summary, these data indicate that the lysine at position 177 restricts the ability of Spry4 to inhibit signal transduction at least in cells with high FGFR1 levels.
Keywords: FGFR1; Spry4; lung cancer; osteosarcoma; sprouty.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Krook M.A., Reeser J.W., Ernst G., Barker H., Wilberding M., Li G., Chen H.Z., Roychowdhury S. Fibroblast growth factor receptors in cancer: Genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br. J. Cancer. 2021;124:880–892. doi: 10.1038/s41416-020-01157-0. - DOI - PMC - PubMed
-
- Domenichini M., Ravelli C., Corsini M., Codenotti S., Moreschi E., Gogna A., Capoferri D., Zizioli D., Bresciani R., Grillo E., et al. The D647N mutation of FGFR1 induces ligand-independent receptor activation. Biochim. Biophys. Acta Gen. Subj. 2023;1867:130470. doi: 10.1016/j.bbagen.2023.130470. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

