Direct and Indirect Downstream Pathways That Regulate Repulsive Guidance Effects of FGF3 on Developing Thalamocortical Axons
- PMID: 40806490
- PMCID: PMC12347462
- DOI: 10.3390/ijms26157361
Direct and Indirect Downstream Pathways That Regulate Repulsive Guidance Effects of FGF3 on Developing Thalamocortical Axons
Abstract
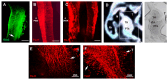
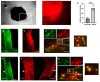
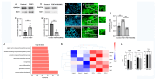
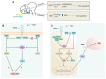
The thalamus is an important sensory relay station. It integrates all somatic sensory pathways (excluding olfaction) and transmits information through thalamic relay neurons before projecting to the cerebral cortex via thalamocortical axons (TCAs). Emerging evidence has shown that FGF3, a member of the morphogen family, is an axon guidance molecule that repels TCAs away from the hypothalamus and into the internal capsule so that they subsequently reach different regions of the cortex. However, current studies on FGF-mediated axon guidance predominantly focus on phenomenological observations, with limited exploration of the underlying molecular mechanisms. To address this gap, we investigated both direct and indirect downstream signaling pathways mediating FGF3-dependent chemorepulsion of TCAs at later developmental stages. Firstly, we used pharmacological inhibitors to identify the signaling cascade(s) responsible for FGF3-triggered direct chemorepulsion of TCAs, in vitro and in vivo. Our results demonstrate that the PC-PLC pathway is required for FGF3 to directly stimulate the asymmetrical repellent growth of developing TCAs. Then, we found the FGF3-mediated repulsion can be indirectly induced by Slit1 because the addition of FGF3 in the culture media induced an increase in Slit1 expression in the diencephalon. Furthermore, by using downstream inhibitors, we found that the indirect repulsive effect of FGF3 is mediated through the PI3K downstream pathway of FGFR1.
Keywords: FGF3; axon guidance; downstream pathways; thalamocortical axons; thalamus.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Reducing health inequalities through general practice: a realist review and action framework.Health Soc Care Deliv Res. 2024 Mar;12(7):1-104. doi: 10.3310/YTWW7032. Health Soc Care Deliv Res. 2024. PMID: 38551093
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

