Circadian Light Manipulation and Melatonin Supplementation Enhance Morphine Antinociception in a Neuropathic Pain Rat Model
- PMID: 40806499
- PMCID: PMC12347545
- DOI: 10.3390/ijms26157372
Circadian Light Manipulation and Melatonin Supplementation Enhance Morphine Antinociception in a Neuropathic Pain Rat Model
Abstract
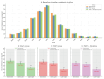
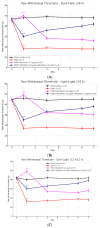
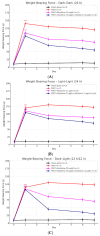
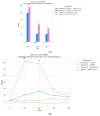
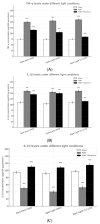
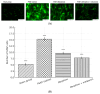
Disruption of circadian rhythms by abnormal light exposure and reduced melatonin secretion has been linked to heightened pain sensitivity and opioid tolerance. This study evaluated how environmental light manipulation and exogenous melatonin supplementation influence pain perception and morphine tolerance in a rat model of neuropathic pain induced by partial sciatic nerve transection (PSNT). Rats were exposed to constant darkness, constant light, or a 12 h/12 h light-dark cycle for one week before PSNT surgery. Behavioral assays and continuous intrathecal (i.t.) infusion of morphine, melatonin, or their combination were conducted over a 7-day period beginning immediately after PSNT. On Day 7, after discontinued drugs infusion, an acute intrathecal morphine challenge (15 µg, i.t.) was administered to assess tolerance expression. Constant light suppressed melatonin levels, exacerbated pain behaviors, and accelerated morphine tolerance. In contrast, circadian-aligned lighting preserved melatonin rhythms and mitigated these effects. Melatonin co-infusion attenuated morphine tolerance and enhanced morphine analgesia. Reduced pro-inflammatory cytokine expression and increase anti-inflammatory cytokine IL-10 level and suppressed astrocyte activation were also observed by melatonin co-infusion during morphine tolerance induction. These findings highlight the potential of melatonin and circadian regulation in improving opioid efficacy and reduced morphine tolerance in managing neuropathic pain.
Keywords: astrocytes; circadian rhythm; light exposure; melatonin; morphine tolerance; neuroinflammation; neuropathic pain; opioid-sparing therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Center for Substance Abuse Treatment . Managing Chronic Pain in Adults with or in Recovery from Substance Use Disorders. Substance Abuse and Mental Health Services Administration (US); Rockville, MD, USA: 2012. SAMHSA/CSAT Treatment Improvement Protocols. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

