Integrating Molecular Alterations with Immunophenotype and Clinical Characteristics in Myelodysplastic Syndromes: A Single-Center Study
- PMID: 40806511
- PMCID: PMC12347109
- DOI: 10.3390/ijms26157382
Integrating Molecular Alterations with Immunophenotype and Clinical Characteristics in Myelodysplastic Syndromes: A Single-Center Study
Abstract
Continuous development of molecular and immunophenotypic techniques enables more precise diagnoses and more accurate assessment of prognosis in myelodysplastic syndromes (MDS). However, the relationship between genetic alterations and immunophenotype remains very poorly understood. The analysis included 30 patients diagnosed at a tertiary center who were eligible for azacitidine treatment. Next-generation sequencing (NGS) was performed at the start of the study to assess the mutation status of 40 genes associated with MDS pathogenesis. In addition, multiparametric flow cytometry (MFC) was performed to assess the ELN score (Ogata score) and, additionally, to detect an abnormal CD11b/HLA-DR and CD11b/CD13 expression pattern. In the studied patient population, higher ELN score results were found in patients with mutations in epigenetic modifiers and pathogenic mutations of the tumor suppressor genes. Signal pathway mutations were associated with lower platelet counts at diagnosis. The results of this study indicate a correlation between molecular abnormalities and deviations in cell immunophenotype. Investigating this correlation may, in the future, allow the development of new scales that allow a more sensitive and specific diagnosis of MDS and a more precise prediction of its course.
Keywords: Flow Cytometry; MDS; MFC; NGS; Ogata score.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
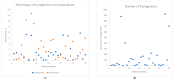


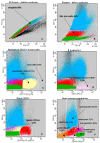

Similar articles
-
The Significance of Detecting an Unusual Myeloblast Immunophenotype in a Presumptive Clinical Diagnosis of Myelodysplastic Syndromes.Arch Pathol Lab Med. 2025 Aug 1;149(8):717-726. doi: 10.5858/arpa.2024-0228-OA. Arch Pathol Lab Med. 2025. PMID: 39711284
-
[Analysis of the association between pre- and post-treatment genetic mutation status and treatment efficacy and survival in patients with newly diagnosed myelodysplastic syndromes with excess blasts receiving hypomethylating agent therapy].Zhonghua Xue Ye Xue Za Zhi. 2025 May 14;46(5):417-424. doi: 10.3760/cma.j.cn121090-20241210-00553. Zhonghua Xue Ye Xue Za Zhi. 2025. PMID: 40623900 Free PMC article. Chinese.
-
Early identification of TP53 mutations and TP53 allelic state in myelodysplastic neoplasms and acute myeloid leukemia via point-of-care p53 immunohistochemistry.Cancer. 2025 Jul 1;131(13):e35950. doi: 10.1002/cncr.35950. Cancer. 2025. PMID: 40542737
-
Thrombopoietin mimetics for patients with myelodysplastic syndromes.Cochrane Database Syst Rev. 2017 Sep 30;9(9):CD009883. doi: 10.1002/14651858.CD009883.pub2. Cochrane Database Syst Rev. 2017. PMID: 28962071 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Cargo C., Bernard E., Beinortas T., Bolton K.L., Glover P., Warren H., Payne D., Ali R., Khan A., Short M. Predicting cytopenias, progression, and survival in patients with clonal cytopenia of undetermined significance: A prospective cohort study. Lancet Haematol. 2024;11:e51–e61. doi: 10.1016/S2352-3026(23)00340-X. - DOI - PubMed
-
- Lee W.H., Lin C.C., Tsai C.H., Tien F.M., Lo M.Y., Tseng M.H., Kuo Y.Y., Yu S.C., Liu M.C., Yuan C.T., et al. ARTICLE Comparison of the 2022 world health organization classification and international consensus classification in myelodysplastic syndromes/neoplasms. Blood Cancer J. 2024;14:57. doi: 10.1038/s41408-024-01031-9. - DOI - PMC - PubMed
-
- Porwit A., Béné M.C., Duetz C., Matarraz S., Oelschlaegel U., Westers T.M., Wagner-Ballon O., Kordasti S., Valent P., Preijers F. Multiparameter flow cytometry in the evaluation of myelodysplasia: Analytical issues: Recommendations from the European LeukemiaNet/International Myelodysplastic Syndrome Flow Cytometry Working Group. Cytom. Part B Clin. Cytom. 2023;104:27–50. doi: 10.1002/cyto.b.22108. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

