Biomaterial-Based Nucleic Acid Delivery Systems for In Situ Tissue Engineering and Regenerative Medicine
- PMID: 40806513
- PMCID: PMC12346993
- DOI: 10.3390/ijms26157384
Biomaterial-Based Nucleic Acid Delivery Systems for In Situ Tissue Engineering and Regenerative Medicine
Abstract
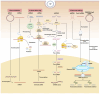
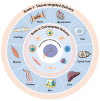
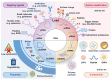
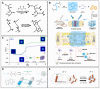
Gene therapy is a groundbreaking strategy in regenerative medicine, enabling precise cellular behavior modulation for tissue repair. In situ nucleic acid delivery systems aim to directly deliver nucleic acids to target cells or tissues to realize localized genetic reprogramming and avoid issues like donor cell dependency and immune rejection. The key to success relies on biomaterial-engineered delivery platforms that ensure tissue-specific targeting and efficient intracellular transport. Viral vectors and non-viral carriers are strategically modified to enhance nucleic acid stability and cellular uptake, and integrate them into injectable or 3D-printed scaffolds. These scaffolds not only control nucleic acid release but also mimic native extracellular microenvironments to support stem cell recruitment and tissue regeneration. This review explores three key aspects: the mechanisms of gene editing in tissue repair; advancements in viral and non-viral vector engineering; and innovations in biomaterial scaffolds, including stimuli-responsive hydrogels and 3D-printed matrices. We evaluate scaffold fabrication methodologies, nucleic acid loading-release kinetics, and their biological impacts. Despite progress in spatiotemporal gene delivery control, challenges remain in balancing vector biocompatibility, manufacturing scalability, and long-term safety. Future research should focus on multifunctional "smart" scaffolds with CRISPR-based editing tools, multi-stimuli responsiveness, and patient-specific designs. This work systematically integrates the latest methodological advances, outlines actionable strategies for future investigations and advances clinical translation perspectives beyond the existing literature.
Keywords: gene therapy; nanoparticle; nucleic acid delivery system; scaffold; vector.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Hydrogel-driven innovations for targeted delivery, immune modulation, and tissue repair in thyroid cancer therapy.Front Cell Dev Biol. 2025 Jul 25;13:1608709. doi: 10.3389/fcell.2025.1608709. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40791984 Free PMC article. Review.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
3D printed metamaterials: properties, fabrication, and drug delivery applications.Adv Drug Deliv Rev. 2025 Sep;224:115636. doi: 10.1016/j.addr.2025.115636. Epub 2025 Jun 9. Adv Drug Deliv Rev. 2025. PMID: 40499618 Review.
-
Trojan Horse-Like Vehicles for CRISPR-Cas Delivery: Engineering Extracellular Vesicles and Virus-Like Particles for Precision Gene Editing in Cystic Fibrosis.Hum Gene Ther. 2025 Aug;36(15-16):1021-1052. doi: 10.1089/hum.2024.258. Epub 2025 Apr 28. Hum Gene Ther. 2025. PMID: 40295092 Review.
-
Hydrogels in Cardiac Surgery: Versatile Platforms for Tissue Repair, Adhesion Prevention, and Localized Therapeutics.Gels. 2025 Jul 21;11(7):564. doi: 10.3390/gels11070564. Gels. 2025. PMID: 40710725 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

