Muscarinic Receptor Antagonism and TRPM3 Activation as Stimulators of Mitochondrial Function and Axonal Repair in Diabetic Sensorimotor Polyneuropathy
- PMID: 40806522
- PMCID: PMC12347734
- DOI: 10.3390/ijms26157393
Muscarinic Receptor Antagonism and TRPM3 Activation as Stimulators of Mitochondrial Function and Axonal Repair in Diabetic Sensorimotor Polyneuropathy
Abstract
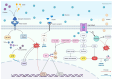
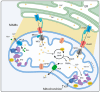
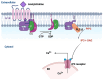
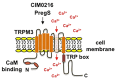
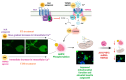
Diabetic sensorimotor polyneuropathy (DSPN) is the most prevalent complication of diabetes, affecting nearly half of all persons with diabetes. It is characterized by nerve degeneration, progressive sensory loss and pain, with increased risk of ulceration and amputation. Despite its high prevalence, disease-modifying treatments for DSPN do not exist. Mitochondrial dysfunction and Ca2+ dyshomeostasis are key contributors to the pathophysiology of DSPN, disrupting neuronal energy homeostasis and initiating axonal degeneration. Recent findings have demonstrated that antagonism of the muscarinic acetylcholine type 1 receptor (M1R) promotes restoration of mitochondrial function and axon repair in various neuropathies, including DSPN, chemotherapy-induced peripheral neuropathy (CIPN) and HIV-associated neuropathy. Pirenzepine, a selective M1R antagonist with a well-established safety profile, is currently under clinical investigation for its potential to reverse neuropathy. The transient receptor potential melastatin-3 (TRPM3) channel, a Ca2+-permeable ion channel, has recently emerged as a downstream effector of G protein-coupled receptor (GPCR) pathways, including M1R. TRPM3 activation enhanced mitochondrial Ca2+ uptake and bioenergetics, promoting axonal sprouting. This review highlights mitochondrial and Ca2+ signaling imbalances in DSPN and presents M1R antagonism and TRPM3 activation as promising neuro-regenerative strategies that shift treatment from symptom control to nerve restoration in diabetic and other peripheral neuropathies.
Keywords: Ca2+ homeostasis; DRG; GPCR; bioenergetics; diabetic neuropathy; pirenzepine.
Conflict of interest statement
The corresponding author, P.F., and co-author N.A.C. declare that they are co-founders of, and shareholders in, WinSanTor Inc., a biotechnology company which has licensed intellectual property from the University of Manitoba and UCSD in the area of antimuscarinic drugs.
Figures

Similar articles
-
Muscarinic acetylcholine type 1 receptor antagonism activates TRPM3 to augment mitochondrial function and drive axonal repair in adult sensory neurons.Mol Metab. 2025 Feb;92:102083. doi: 10.1016/j.molmet.2024.102083. Epub 2024 Dec 16. Mol Metab. 2025. PMID: 39694091 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Redefining distal symmetrical polyneuropathy features in type 1 diabetes: a systematic review.Acta Diabetol. 2022 Jan;59(1):1-19. doi: 10.1007/s00592-021-01767-x. Epub 2021 Jul 2. Acta Diabetol. 2022. PMID: 34213655 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

