Identification of Common Cancer Antigens Useful for Specific Immunotherapies to Colorectal Cancer and Liver Metastases
- PMID: 40806530
- PMCID: PMC12347713
- DOI: 10.3390/ijms26157402
Identification of Common Cancer Antigens Useful for Specific Immunotherapies to Colorectal Cancer and Liver Metastases
Abstract
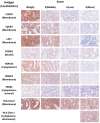
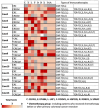
Stage IV colorectal cancer has a poor prognosis, and liver metastases are prone to recurrence, even after resection. This study aimed to identify common cancer antigens, using immunohistochemical staining, as promising targets for antigen-specific immunotherapies in colorectal cancer. We analyzed expression levels and intracellular localization of seven common cancer antigens, CLDN1, EphB4, LAT1, FOXM1, HSP105α, ROBO1, and SPARC, and human leukocyte antigen (HLA) class I via immunohistochemical staining of 85 surgical specimens from primaries and liver metastases. Staining intensity and positive staining were scored to evaluate antigen expression. In 25 primaries, seven cancer antigens were expressed in 88-96% of cases, while HLA class I was expressed on the cell membrane in 80.0% of cases. In 60 liver metastases, FOXM1 and SPARC expression were approximately half that observed in the primaries. Other antigens and HLA class I were highly expressed in both. Most of the primaries and liver metastases may benefit from chimeric antigen receptor-T cell therapy targeting CLDN1, EphB4, and LAT1. Cases with high HLA class I expression may be suitable for vaccine-based and T cell receptor-T cell therapy targeting CLDN1, EphB4, LAT1, FOXM1, HSP105α, ROBO1, and SPARC for primaries and targeting antigens, excluding FOXM1 and SPARC, for liver metastases.
Keywords: T cell receptor-T (TCR-T) cell therapy; cancer vaccine; chimeric antigen receptor-T (CAR-T) cell therapy; human leukocyte antigen class I; immunohistochemical staining.
Conflict of interest statement
Tetsuya Nakatsura (TN) received a research grant from BrightPath Biotherapeutics Co., Ltd.; Thyas Co., Ltd.; ONOPHARMACEUTICAL CO., Ltd.; Resonac Corporation; MEDINET Co., Ltd.; NapaJen Pharma Inc.; Heartseed Inc.; Takara Biio Inc.; DAICEL CORPORATION; NA Vaccine Institute CO., Ltd.; Logomix Inc.; Optieum Biotechnologies Inc.; and MaxCyte, Inc. TN holds stock ownership, stock option, or profits from Noile-Immune Biotech Inc., Logomix Inc., and Optieum Biotechnologies Inc. TN also has royalties from OncoTherapyScience, Inc. The other authors have no conflicts of interest to declare.
Figures


Similar articles
-
Identification of 68 HLA-A24 and -A2-restricted cytotoxic T lymphocyte-inducing peptides derived from 10 common cancer-specific antigens frequently expressed in various solid cancers.Neoplasia. 2025 Mar;61:101135. doi: 10.1016/j.neo.2025.101135. Epub 2025 Feb 11. Neoplasia. 2025. PMID: 39938154 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent.Cochrane Database Syst Rev. 2017 Dec 16;12(12):CD011300. doi: 10.1002/14651858.CD011300.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Dec 6;12:CD011300. doi: 10.1002/14651858.CD011300.pub3. PMID: 29247502 Free PMC article. Updated.
-
Transarterial (chemo)embolisation versus systemic chemotherapy for colorectal cancer liver metastases.Cochrane Database Syst Rev. 2024 Aug 9;8(8):CD012757. doi: 10.1002/14651858.CD012757.pub2. Cochrane Database Syst Rev. 2024. PMID: 39119869 Free PMC article. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Tsukamoto F., Tsukamoto S., Kato T., Nagata H., Takamizawa Y., Moritani K., Kinugasa Y., Esaki M., Kanemitsu Y., Igarashi A. Cost-effectiveness analysis of postoperative surveillance for stage IV colorectal cancer in Japan: An economic modeling study. Ann Gastroenterol Surg. 2025;9:730–738. doi: 10.1002/ags3.12906. - DOI - PMC - PubMed
-
- Adam R., de Gramont A., Figueras J., Kokudo N., Kunstlinger F., Loyer E., Poston G., Rougier P., Rubbia-Brandt L., Sobrero A., et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015;41:729–741. doi: 10.1016/j.ctrv.2015.06.006. - DOI - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Colon Cancer Version 5.2024. [(accessed on 22 August 2024)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

