A Conserved N-Terminal Di-Arginine Motif Stabilizes Plant DGAT1 and Modulates Lipid Droplet Organization
- PMID: 40806535
- PMCID: PMC12347784
- DOI: 10.3390/ijms26157406
A Conserved N-Terminal Di-Arginine Motif Stabilizes Plant DGAT1 and Modulates Lipid Droplet Organization
Abstract
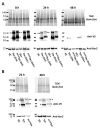
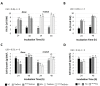
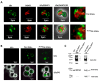
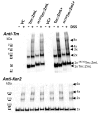
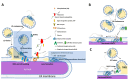
Diacylglycerol-O-acyltransferase 1 (DGAT1, EC 2.3.1.20) is a pivotal enzyme in plant triacylglycerol (TAG) biosynthesis. Previous work identified conserved di-arginine (R) motifs (R-R, R-X-R, and R-X-X-R) in its N-terminal cytoplasmic acyl-CoA binding domain. To elucidate their functional significance, we engineered R-rich sequences in the N-termini of Tropaeolum majus and Zea mays DGAT1s. Comparative analysis with their respective non-mutant constructs showed that deleting or substituting R with glycine in the N-terminal region of DGAT1 markedly reduced lipid accumulation in both Camelina sativa seeds and Saccharomyces cerevisiae cells. Immunofluorescence imaging revealed co-localization of non-mutant and R-substituted DGAT1 with lipid droplets (LDs). However, disruption of an N-terminal di-R motif destabilizes DGAT1, alters LD organization, and impairs recombinant oleosin retention on LDs. Further evidence suggests that the di-R motif mediates DGAT1 retrieval from LDs to the endoplasmic reticulum (ER), implicating its role in dynamic LD-ER protein trafficking. These findings establish the conserved di-R motifs as important regulators of DGAT1 function and LD dynamics, offering insights for the engineering of oil content in diverse biological systems.
Keywords: Camelina; DGAT1; di-arginine motif; lipid droplets; oleosin; transmembrane protein; yeast.
Conflict of interest statement
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
APOE4 alters the lipid droplet proteome and modulates droplet dynamics.bioRxiv [Preprint]. 2024 Dec 20:2024.12.16.628710. doi: 10.1101/2024.12.16.628710. bioRxiv. 2024. Update in: Neurobiol Dis. 2025 Aug;212:106983. doi: 10.1016/j.nbd.2025.106983. PMID: 39763832 Free PMC article. Updated. Preprint.
-
DGAT1 Inhibition Enhances Olaparib-Induced Lipotoxic Apoptosis in Metastatic Castration-Resistant Prostate Cancer.FASEB J. 2025 Aug 15;39(15):e70925. doi: 10.1096/fj.202501046R. FASEB J. 2025. PMID: 40788201
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Pharmacological treatment of children with gastro-oesophageal reflux.Cochrane Database Syst Rev. 2014 Nov 24;2014(11):CD008550. doi: 10.1002/14651858.CD008550.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2023 Aug 22;8:CD008550. doi: 10.1002/14651858.CD008550.pub3. PMID: 25419906 Free PMC article. Updated.
-
Lipid Droplets' Role in the Regulation of β-Cell Function and β-Cell Demise in Type 2 Diabetes.Endocrinology. 2022 Mar 1;163(3):bqac007. doi: 10.1210/endocr/bqac007. Endocrinology. 2022. PMID: 35086144 Free PMC article. Review.
References
-
- Murphy D. Biotechnology and the improvement of oil crops—Genes, dreams and realities. Phytochem. Rev. 2022;1:67–77. doi: 10.1023/A:1015884319559. - DOI
-
- Ohlrogge J., Allen D., Berguson B., Dellapenna D., Shachar-Hill Y., Stymne S. Driving on biomass: Burning biomass to produce electricity for battery-driven vehicles can power more travel and displace more petroleum than converting it to ethanol or other fermentation products. Science. 2009;324:1019–1020. doi: 10.1126/science.1171740. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

