APOE Genotyping in Cognitive Disorders: Preliminary Observations from the Greek Population
- PMID: 40806539
- PMCID: PMC12347155
- DOI: 10.3390/ijms26157410
APOE Genotyping in Cognitive Disorders: Preliminary Observations from the Greek Population
Abstract
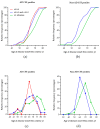
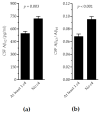
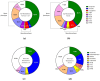
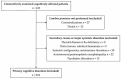
Alzheimer's disease (AD) is the most common cause of cognitive decline. Among the various susceptibility genes, the gene of apolipoprotein E (APOE) is probably the most important. It may be present in three allelic forms, termed ε2, ε3 and ε4, and the most common genotype is the ε3/ε3. Recently, it has been observed that subjects with the ε4/ε4 genotype may show near-full penetrance of AD biology (pathology and biomarkers), leading to the suggestion that ε4 homozygosity may represent a distinct genetic type of AD. The aim of the present study was to investigate the role of ε4 homozygosity or heterozygosity in the presence or absence of the AD biomarker profile in patients with cognitive disorders in the Greek population. A total of 274 patients were included in the study. They underwent APOE genotyping and cerebrospinal fluid (CSF) biomarker profiling. The presence of ε4 was associated with a lower age of symptom onset and decreased amyloid biomarkers (irrespective to AD or non-AD profiles), and predicted the presence of an AD profile by a positive predictive value approaching 100%. In conclusion, the ε4 allele has a significant effect on the risk and clinical parameters of cognitive impairment and AD in the Greek population, while the ε4/ε4 genotype may be highly indicative of the (co)existence of AD in cognitively impaired patients.
Keywords: Alzheimer’s disease; amyloid beta; apolipoprotein E; cognitive impairment; dementia; phospho-tau protein; tau protein.
Conflict of interest statement
G.T., G.P.P., I.T, A.A., C.Z. and A.B. are clinical investigators in the “EVOKE” and “EVOKE plus” trials of semaglutide for early Alzheimer’s disease (NovoNordisk, NCT04777396 and NCT04777409, respectively). G.P.P received fees from Biogen International and from ITF Hellas, as a consultant of advisory boards. C.K. declares fees from Roche, Abbott and Snibe. S.J.T has shares in the research and diagnosis laboratory Tzartos NeuroDiagnostics. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Dubois B., Villain N., Frisoni G.B., Rabinovici G.D., Sabbagh M., Cappa S., Bejanin A., Bombois S., Epelbaum S., Teichmann M., et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021;20:484–496. doi: 10.1016/S1474-4422(21)00066-1. - DOI - PMC - PubMed
-
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

